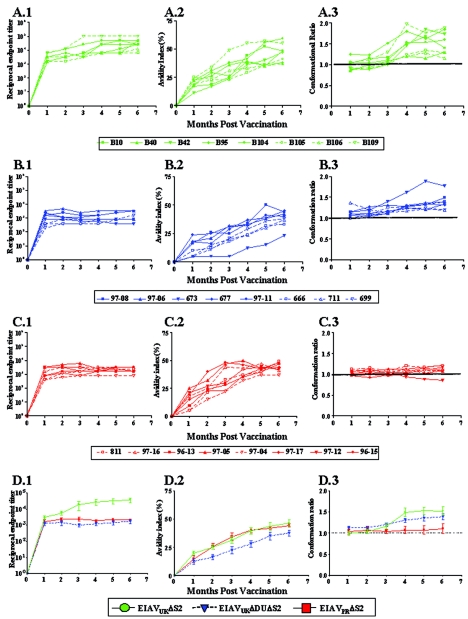
FIG. 5.
Development of envelope-specific antibody responses to various attenuated EIAV vaccines. Longitudinal characterization of the quantitative and qualitative properties of induced EIAV envelope-specific antibodies were conducted in concanavalin A ELISAs of endpoint titer (panels 1), avidity (panels 2), and conformational dependence (panels 3) as described in Materials and Methods. Analyses were performed on allthree trial groups: EIAVUKΔS2 (A), EIAVUKΔS2/DU (B), and EIAVPRΔS2 (C). (D) Average levels for each assessment were also calculated to extricate and simplify comparative analysis. (A.1 to D.1) Antibody titers in serum for each time point are presented as the log10 of the highest reciprocal dilution yielding reactivity two standard deviations above background. (A.2 to D.2) Avidity index measurements are presented as percentages of the antibody-antigen complexes resistant to disruption with 8 M urea. (A.3 to D.3) Conformation dependence values are calculated as the ratio of serum antibody reactivity with native envelope compared to denatured envelope antigen. Conformation ratios of >1.0 indicate predominant antibody specificity for conformational determinants, whereas ratios of <1.0 indicate predominant antibody specificity for linear envelope determinants.