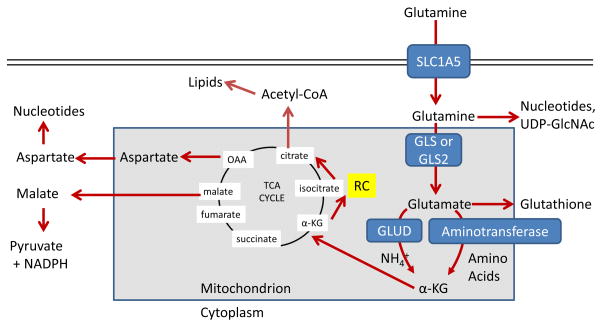
Figure 2. Major metabolic and biosynthetic fates of glutamine.
Glutamine enters the mammalian cell through transporters such as SLC1A5 (also known as ASCT2) 15. Glutamine itself can contribute to nucleotide biosynthesis and uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) synthesis for support of protein folding and trafficking 210, or is converted to glutamate by glutaminase (GLS or GLS2) 28. Glutamate can contribute to the synthesis of glutathione 110, and has many other metabolic fates in the cell that impact on several inborn errors of metabolism, which were recently reviewed 211. Glutamate is converted to α-ketoglutarate (αKG) through one of two sets of enzymes, glutamate dehydrogenase (GLUD1 or GLUD2, henceforth referred to collectively as GLUD) or aminotransferases 30. While the byproduct of GLUD is NH4+, the byproduct of aminotransferase reactions is other amino acids. Note that aminotransferases may be present either in the cytoplasm or the mitochondria. α-ketoglutarate enters the tricarboxylic acid (TCA) cycle and can provide energy for the cell. Malate exiting the TCA cycle can produce pyruvate and NADPH for reducing equivalents 31, and oxaloacetate (OAA) can be converted to aspartate to support nucleotide synthesis 34. These two pathways are illustrated in more detail in Figure 4. Alternately, α-KG can proceed backwards through the TCA cycle, in a process called reductive carboxylation (RC) to produce citrate, which supports synthesis of acetyl-CoA and lipids 87.