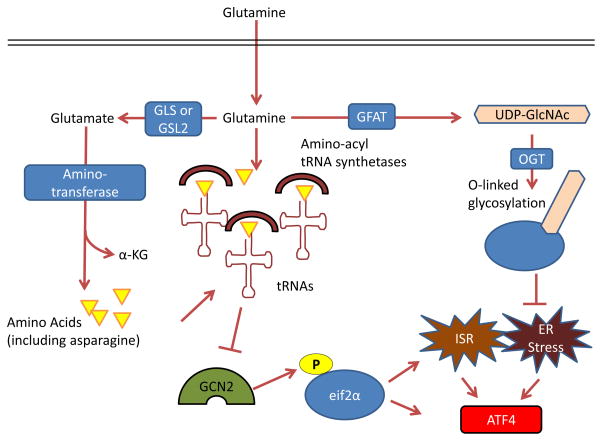
Figure 4. Control by glutamine of the integrated stress response, protein folding and trafficking, and ER stress.
GCN2, a serine-threonine kinase with a regulatory domain that is structurally similar to histidine-tRNA synthetase, is allosterically activated by uncharged tRNAs with amino acid deprivation (including glutamine deprivation) and in turn activates the integrated stress response (ISR) 96, 214, 215. Glutamine can suppress GCN2 activation through its contribution to amino acid pools by aminotransferases 65, 97–99. To control endoplasmic reticulum (ER) homeostasis, glutamine supports protein folding and trafficking through its contribution to uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as part of the hexosamine biosynthesis pathway. Glutamine is the substrate for glutamine fructose-6-phosphate aminotransferase (GFAT), which is the key rate-limiting enzyme in the hexosamine pathway, and the downstream product UDP-GlcNAc is a substrate for O-linked glycosylation through O-linked β-N-acetylglucosamine transferase (OGT). Thus, glutamine deprivation can lead to improper protein folding and chaperoning and ER stress 210. A key output of both the ISR and of ER stress is activating transcription factor 4 (ATF4), which is induced via cap-independent translation downstream of eukaryotic translation initiation factor 2α (eIF2α) phosphorylation by GCN2 or other kinases 96. α-KG, α-ketoglutarate; GLS, kidney-type glutaminase; GLS2, liver-type glutaminase.