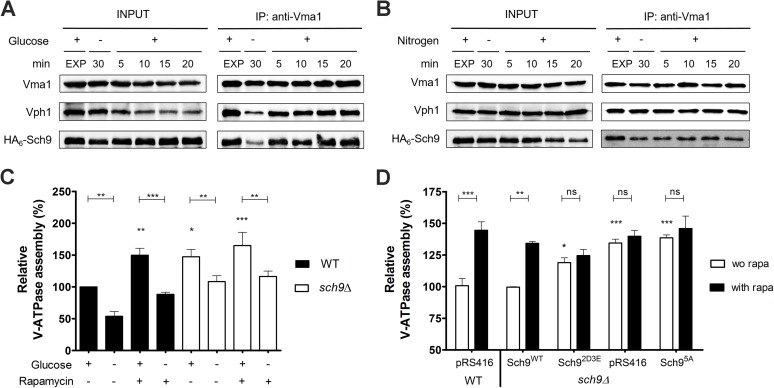
Fig 7. Sch9 physically interacts with the V-ATPase.
(A, B) Physical interaction of Sch9 with Vma1 depends on glucose availability. Cells expressing HA6-Sch9 were grown as in Fig 6A, followed by re-addition of 2% glucose (A) or 0.2% glutamine (B). Total lysates (input) and anti-Vma1 immunoprecipitates (IP) were analyzed by immunoblotting. (C, D) Sch9 regulates V-ATPase assembly downstream of TORC1. (C) WT and sch9Δ cells were grown to mid-log phase in YPD, pH 5. Half of the culture was treated with 200 nM rapamycin for 30 min and subsequently starved for glucose in the presence of rapamycin. The untreated half was further grown for 30 min and subsequently starved for glucose. (D) V-ATPase assembly was assessed in the sch9Δ strain expressing the empty vector (pRS416), the wild-type SCH9 gene (Sch9WT), or one of the SCH9 mutant genes in which its TORC1 phosphorylation sites are mutated (Sch95A and Sch92D3E). The WT strain expressing the empty vector was taken as an additional control. Precultures were grown overnight in minimal medium lacking uracil buffered at pH 5 and inoculated in YPD medium (50 mM MES, pH 5). Once cells reached exponential phase, half of the culture was treated with 200 nM rapamycin (rapa) for 30 min. To quantify V-ATPase assembly, complexes were IPed with antibodies against Vma1 and Vph1. Results are depicted as mean values ± SEM from at least three independent experiments. One- or two-way ANOVA analyses were performed to determine statistical significances. Unless indicated otherwise, asterisks indicate a statistical significance compared to the WT strain grown in YPD without rapamycin. See also Table 2.