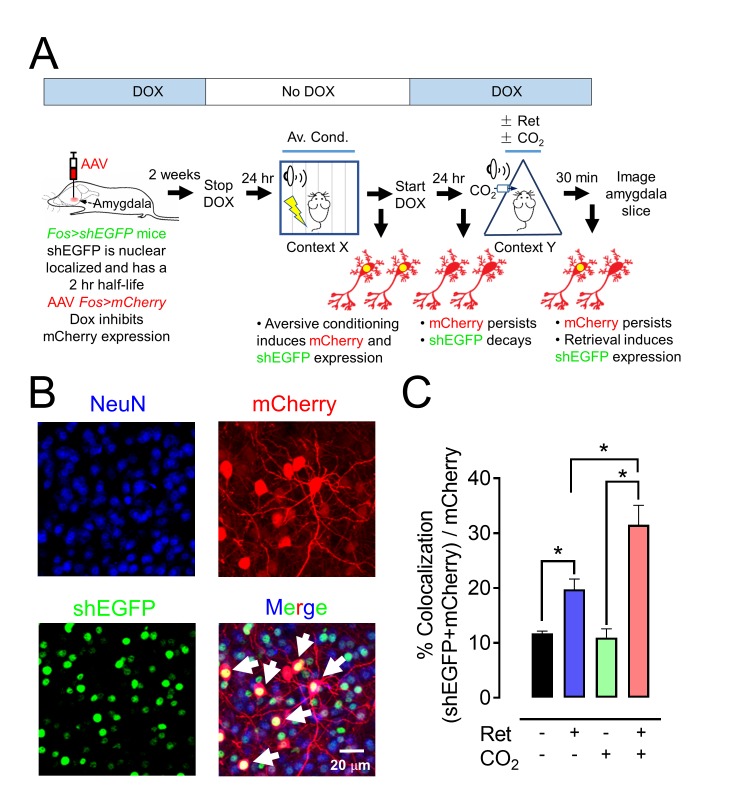
Figure 7. CO2 inhalation enhances retrieval-induced activation of lateral amygdala neurons bearing an aversive memory trace.
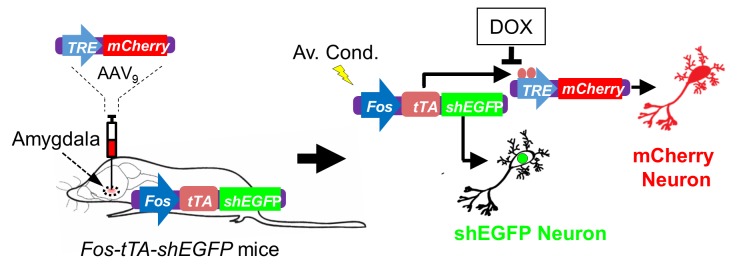
(A) Schematic showing procedure; see also Figure 7—figure supplement 1 for additional information about the doxycycline-inducible system. The activity-dependent promoter Fos was used to induce expression of nuclear localized shEGFP in transgenic mice, indicated as Fos>shEGFP. shEGFP rapidly decays with a half-life of 2 hr. Mice were fed doxycycline (DOX) for 1 week, and then an AAV9 vector encoding mCherry, indicated as Fos>mCherry (which is ultimately expressed when the Fos promoter is activated), was microinjected into the amygdala. DOX inhibits mCherry expression. Two weeks later, DOX was discontinued, and mice underwent the aversive conditioning protocol as described in Figure 1A. Under those conditions, the Fos promoter will drive both shEGFP and mCherry expression, but shEGFP will rapidly decay. After aversive conditioning, mice immediately resumed DOX treatment. One day later, the mice underwent the Ret/CO2 protocol shown in Figure 1A. Thirty minutes after that, brain slices were prepared and the shEGFP- and mCherry-positive neurons in the lateral amygdala were determined. (B) Example of lateral amygdala neurons labeled by mCherry (red), the nuclear localized shEGFP (green), and NeuN (blue). White arrows indicate examples of colocalization of mCherry and shEGFP. mCherry- and shEGFP-positive cells were also NeuN-positive. (C) Data are the percentage of mCherry-positive cells that were also shEGFP-positive. Data are mean ±SEM. n = 5 mice for each group. * indicates p<0.05 by ANOVA with Tukey’s post hoc multiple comparison. In C, No ret vs Ret, p=0.0154; No ret vs No ret + CO2, p=0.9915; No ret vs Ret + CO2, p<0.0001; Ret vs No ret + CO2, p=0.0197; Ret vs Ret + CO2, p=0.0013; No ret + CO2 vs Ret + CO2, p<0.0001.