Figure 5.
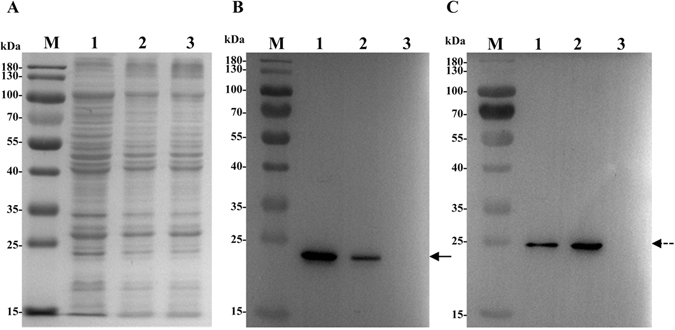
Determination of UmpA and UmpB as a hetero-dimer by co-immunoprecipitation. For the determination of UmpA and UmpB as a hetero-dimer, the stored membrane fraction containing 1.2 mg total membrane protein from E. coli KNabc cells carrying pETDuet-1-umpAB were re-suspended in an ice-cold commercially-available buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100 and a certain amount of protease inhibitors including sodium pyrophosphate, β-glycerophosphate, EDTA, sodium ortovanadate and leupeptin. And then the primary antibody [a rabbit anti-His6-tag antibody (Abcom Ltd, China) for UmpA] was added to the solubilized membrane proteins and precipitated with protein agarose A-sepharose. After washing three times with the ice-cold above-mentioned commercially-available buffer, the precipitates (Lane 2), together with membrane fraction (as a positive control, Lane 1) and the rabbit IgG mixed with the solubilized membrane proteins by the protein agarose A-sepharose (as a negative control, Lane 3), were used for the SDS-PAGE (A) and western blots. To avoid the visualization of the light chain and the heavy chain from a rabbit anti-His6-tag antibody in the precipitates, His6-tag detection (B) was done by using a mouse anti-His6-tag antibody and a goat anti-mouse horseradish peroxidase-labelled secondary antibody. c-Myc-tag detection (C) was done by using a mouse anti-c-Myc-tag antibody and a goat anti-mouse horseradish peroxidase-labelled secondary antibody. The positions of target proteins, UmpA fused with a N-terminal His6 tag and UmpB fused with a C-terminal c-Myc tag, are shown with a solid line arrow and a dotted line arrow, respectively.
