Figure 3.
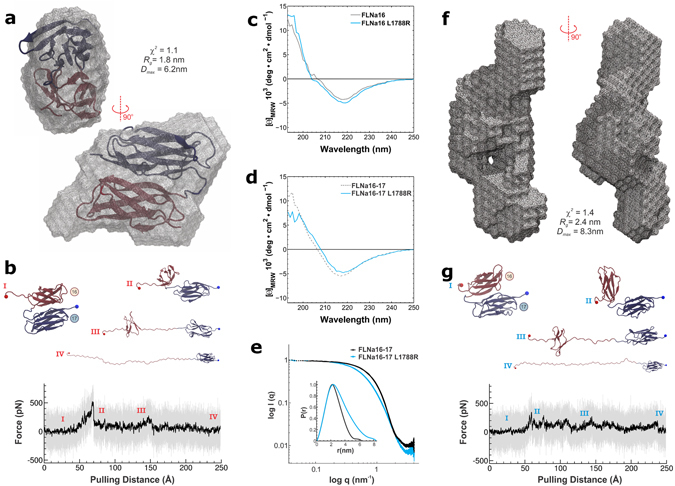
Effects of FMD associated mutation on the structure of FLNa16-17. (a) The ab initio shape envelopes calculated form SAXS data shows that FLNa16-17 WT is globular in accordance with the NMR-structure of FLNa16-1722, which is superimposed with the SAXS envelope. The molecular dimensions in terms of R g (radius of gyration) and D max (maximum dimensions), as well as, the fit of the shape envelope on the experimental scattering curve (χ2) are shown. (b) Unfolding trace of FLNa16-17 WT obtained from SMD a pulling speed of 2.5 Å ns−1 shows that interaction between domains 16 and 17 is lost first, followed by domain 16 unfolding. The full trajectory is shown gray. The black line represents a moving average with a box size of 500 steps. The snapshots of different time steps are labelled I-IV. (c,d). The CD-spectroscopy analyses of the WT and mutated FLNa16 (c) and FLNa16-17 (d) shows that L1788R does not destroy the β-sheet folding of FLNa16. (e,f). The experimental scattering data, the distance-distribution function, P(r), (insert), and the ab initio shape envelope obtained from SAXS measurements show that WT FLNa16-17 (panel A) is globular but L1788R mutated FLNa16-17 is elongated (f). The scattering data shown in e is scaled to the same forward scattering intensity I(0). The molecular dimensions of the L1788R envelope in terms of R g and D max, as well as, (χ2) are shown in (f). (g) The unfolding trace of FLNa16-17 L1788R obtained from SMD a pulling speed of 2.5 Å ns−1 shows that similarly As with WT, the interaction between domains 16 and 17 is lost first, followed by domain 16 unfolding. However, the force needed to unfold L1788R mutated FLNa16-17 is notably lower than that needed for WT. The full trajectory is shown gray. The black line represents a moving average with a box size of 500 steps. The snapshots of different time steps are labelled I-IV.
