Figure 5.
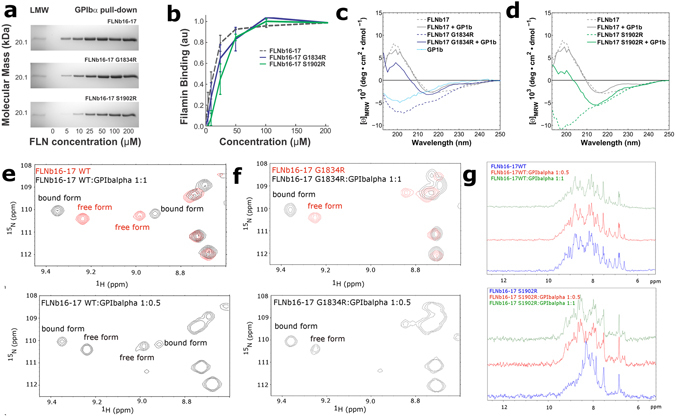
Effects of G1834R and S1902R mutations on FLNb16-17 interactions. (a,b) The binding assays show that FLNb16-17 G1834R and S1902R mutants are able to bind the GP1bα-peptide almost similar avidity as FLNb16-17 WT despite of the fact that these mutation unfold domain 17. GP1bα-peptide binding to FLNb16-17 WT and G1834R and S1902R mutants in 5, 10, 25, 50, 100, and 200 μM concentrations is shown. FLNb16-17 WT and G1834R and S1902R mutants binding to GP1bα-peptide was quantified by protein staining and expressed as FLN binding (in arbitrary units) calculated as the ratio of FLN bound to FLN in the loading control, normalized to maximal FLN binding in each experiment (mean S.E. (error bars); n ≥ 3). (c,d) The CD-spectrometry measurements show that the GP1bα-peptide binding to G1834R (c) and S1902R (d) mutated FLNb17 induces β-sheet folding on these domains. (e,f) Selected regions of HSQC spectra of 15N-labelled FLNb16-17 WT and G1834R fragments collected before and after titration with GP1bα-peptide shows tight binding of the peptide to both WT (e) and G1834R (f) fragments. (g) One-dimensional 15N-edited, 1H spectra of FLNb16-17 WT and S1902R with increasing concentration of GPIbα peptide. Spectra for S1902R mutant highlight increasing dispersion of amide proton chemical shifts upon titration of GPIbα-peptide, indicative of growing structural order.
