Figure 3.
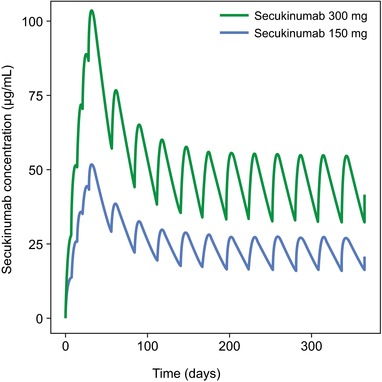
Simulated concentration profiles of secukinumab 300 and 150 mg with subcutaneous dosing regimens derived from phase 3 trials. Patients were simulated to receive secukinumab at baseline; weeks 1, 2, and 3; and then every 4 weeks from week 4 to week 48.
