Figure 4.
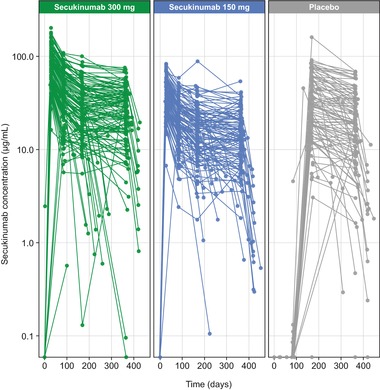
Observed serum secukinumab concentrations. Each line represents serum secukinumab concentrations from an individual patient in the phase 3 ERASURE trial.9 Secukinumab was administered at baseline; weeks 1, 2, and 3; and then every 4 weeks from week 4 to week 48. The last dose of secukinumab was given at week 48. Patients receiving placebo who did not achieve PASI 75 at week 12 were rerandomized to secukinumab 300 or 150 mg. Pharmacokinetic analysis was performed at baseline; weeks 4, 12, 24, 52, and 60; and during unscheduled visits.
