Figure 5.
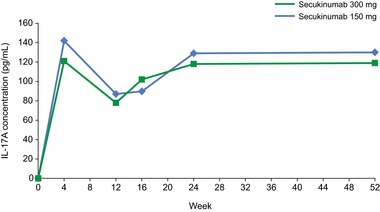
Observed serum total I‐17A concentrations. Secukinumab was administered at baseline; weeks 1, 2, and 3; and then every 4 weeks from week 4 to week 48. Pharmacodynamic analysis was performed at baseline and weeks 4, 12, 16, 24, and 52. Results represent median total IL‐17A concentrations from patients in the phase 3 JUNCTURE trial.13
