Summary
Aims/objectives:
In the BESIDE study, combination therapy (antimuscarinic [solifenacin] and β3‐adrenoceptor agonist [mirabegron]) improved efficacy over solifenacin monotherapy without exacerbating anticholinergic side effects in overactive bladder (OAB) patients; however, a potential synergistic effect on the cardiovascular (CV) system requires investigation.
Methods
OAB patients remaining incontinent despite daily solifenacin 5 mg during 4‐week single‐blind run‐in, were randomised 1:1:1 to double‐blind daily combination (solifenacin 5 mg/mirabegron 25 mg, increasing to 50 mg after week 4), solifenacin 5 or 10 mg for 12 weeks. CV safety assessments included frequency of CV‐related treatment‐emergent adverse events (TEAEs), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure [DBP], pulse rate) and electrocardiogram (ECG) parameters.
Results
The frequency of hypertension, tachycardia and ECG QT prolongation, respectively, was low and comparable across combination (1.1%, 0.3%, 0.1%), solifenacin 5 mg (0.7%, 0.1%, 0.1%), and solifenacin 10 mg groups (0.8%, 0%, 0.1%). Adjusted mean (SE) change from baseline to end of treatment (EoT) in SBP, DBP, and pulse rate with combination (0.07 mm Hg [0.38], −0.35 mm Hg [0.26], 0.47 bpm [0.28]), solifenacin 5 mg (−0.93 mm Hg [0.38], −0.45 mm Hg [0.26], 0.43 bpm [0.28]) and solifenacin 10 mg (−1.28 mm Hg [0.38], −0.48 mm Hg [0.26], 0.27 bpm [0.28]) was generally comparable, with the exception of a mean treatment difference of ~1 mm Hg in SBP between combination and solifenacin monotherapy; SBP was unchanged with combination and decreased with solifenacin monotherapy. Mean changes from baseline to EoT in ECG parameters were generally similar across treatment groups, except for QT interval corrected using Fridericia's formula, which was higher with solifenacin 10 mg (3.30 mseconds) vs. combination (0.49 mseconds) and solifenacin 5 mg (0.77 mseconds).
Conclusion
The comparable frequency of CV‐related TEAEs, changes in vital signs and ECG parameters indicates no synergistic effect on CV safety outcomes when mirabegron and solifenacin are combined.
What's known
A combination of antimuscarinic (solifenacin 5 mg) and β3‐adrenoceptor agonist (mirabegron 50 mg) improves efficacy without exacerbating anticholinergic side effects vs. solifenacin monotherapy (5 mg, 10 mg) in the treatment of overactive bladder (OAB).
Cardiovascular (CV) comorbidities are more prevalent in OAB patients compared with the non‐OAB population.
Mirabegron and solifenacin in combination may potentially affect the CV system; however, monotherapy use at therapeutic doses indicates no CV safety concerns.
What's new
Using a combination of mirabegron and solifenacin does not have a synergistic effect on the CV system since the frequency of CV‐related treatment‐emergent adverse events, change in vital signs and ECG parameters are comparable with solifenacin monotherapy (5 mg and 10 mg).
1. Introduction
The symptom complex of overactive bladder (OAB) is defined as urinary urgency, often accompanied by increased daytime micturition frequency and nocturia in the absence of urinary tract infection or other obvious pathology; urgency incontinence may or may not be present.1, 2 OAB is estimated to affect 20% of the global population by 2018.3 Increasing age is the most common risk factor for OAB, with current prevalence rates of 30%‐40% in patients aged ≥65 years.4, 5 Males and females are equally affected, however, certain symptoms predominate in males (urgency and nocturia) and females (incontinence).6, 7 Since OAB is an age‐related condition, significantly more patients present with concomitant cardiovascular (CV) comorbidities (eg, hypertension) compared with non‐OAB patients,8 which emphasises the importance of evaluating the CV safety of OAB pharmacotherapies.
The two available oral pharmacotherapies—antimuscarinics and the β3‐adrenoceptor agonist, mirabegron—mediate relaxation of the bladder by antagonism of the muscarinic M2 and M3 receptor subtypes,9 or stimulation of the β3‐adrenoceptor subtype, in the urothelium and detrusor muscle.10 Muscarinic (M2, M3) receptors and β‐adrenoceptors (β1, β2 and β3) are also expressed in the CV system. The M2 receptor has a functional role in mediating heart rate11 and the M3 receptor mediates vasodilation,12 and their antagonism could potentially increase blood pressure or heart rate, prolong the QT interval and induce polymorphic ventricular tachycardia (torsade de pointes).11 The β1‐adrenoceptor mediates increased heart rate and force of contraction and the β2‐adrenoceptor mediates vasodilation in the vascular smooth muscle.13 The role of the β3‐adrenoceptor is less clear in human physiology, however, in vitro, the activation of the β3‐adrenoceptor induces positive inotropic effects in human atrial tissue and negative inotropic effects in ventricular tissue.14 The antimuscarinic, solifenacin, is selective for the M3 subtype,15 while in‐vitro studies show that mirabegron has a 150‐fold and 33‐fold higher affinity for the β3‐ vs. β1‐ and β2‐adrenoceptor subtypes.16 Given the location of the M3 receptor and β3 adrenoceptor in CV tissues, and the high density of β1 adrenoceptors in the heart, potential effects on the heart and vasculature cannot be excluded when these drugs are used as monotherapy or in combination.
Mirabegron and solifenacin share similar efficacy in the treatment of OAB,17 and are considered to have an acceptable CV safety profile at therapeutic doses.18, 19 As per mirabegron's labelling, periodic blood pressure monitoring is advocated during treatment, and mirabegron is not recommended in patients with severe uncontrolled hypertension (systolic blood pressure [SBP] ≥180 mm Hg and/or diastolic blood pressure [DBP] ≥110 mm Hg).20 Given the lack of antimuscarinic and mirabegron data in older patients with significant CV risk factors in Phase III trials, it is considered good clinical practice to periodically monitor blood pressure and heart rate in OAB patients aged >80 years.18, 19
OAB patients are usually initiated on an antimuscarinic, however, persistence is often poor because of bothersome anticholinergic side effects (eg, blurred vision, dry mouth) or inadequate improvement in symptoms.21, 22 Switching to an alternative antimuscarinic usually has little impact in terms of improved persistence,23 while dose escalation can often exacerbate the anticholinergic burden leading to treatment discontinuation.21, 24
Several OAB trials investigating combinations of solifenacin (2.5/5/10 mg) and mirabegron (25/50 mg) have demonstrated an additive benefit in efficacy without compromising safety vs. solifenacin monotherapy.25, 26, 27 In the BESIDE study (NCT01908829), adding mirabegron 50 mg to solifenacin 5 mg further improved OAB symptoms and patient‐reported outcomes vs. solifenacin 5 mg or 10 mg, and was well‐tolerated in OAB patients remaining incontinent after initial solifenacin 5 mg.25, 28 Herein, we report on the CV safety outcomes from BESIDE including subpopulations stratified by hypertensive status and gender (age‐related CV safety will be reported in a subsequent manuscript).
2. Methods
2.1. Study design and participants
The methodology for BESIDE has been previously described.25 In summary, adults with OAB symptoms for ≥3 months entered a 2‐week screening/wash‐out period, followed by a run‐in period with single‐blind daily solifenacin 5 mg for 4 weeks provided they reported an average of ≥2 incontinence episodes/24 h prior to the run‐in period. Patients remaining incontinent at baseline (≥1 episode during the 3‐day bladder diary), who satisfied inclusion, and did not meet exclusion criteria, were randomised 1:1:1‐12 weeks of daily double‐blind treatment with combination (solifenacin 5 mg/mirabegron 25 mg, increasing to 50 mg after week 4), solifenacin 5 mg or 10 mg monotherapy.
2.2. CV safety assessment
The frequency of CV‐related treatment‐emergent adverse events (TEAEs) was assessed during the study and 2‐week follow‐up and included increased blood pressure, QT prolongation, increased heart rate, tachycardia, atrial fibrillation, and palpitations. Potentially serious CV‐related events were adjudicated by the Independent Cardiovascular Adjudication Committee and were summarised by type of CV event (Antiplatelet Trialists’ Collaboration [APTC]/Major Adverse Cardiovascular Events [MACE] or non‐APTC/MACE) and non‐CV event.
For vital signs (SBP, DBP, and pulse rate), triplicate readings were measured on site at screening, randomisation (baseline), and each follow‐up visit and the average of the last two readings was used to derive the average per analysis visit. The change from baseline to end of treatment (EoT) in vital signs in the overall population, and according to subpopulations based on hypertension status at screening and β‐blocker use during the treatment period were previously presented in the primary analysis of BESIDE,25 and the adjusted change from baseline to EoT values are summarised in this analysis. Additional sensitivity analyses included a factor for antihypertensive use at screening or a different age group factor (<45, ≥45 to <65, ≥65 years) in the model. Change in vital signs was also stratified by gender. Shift in vital sign severity according to four categories of average blood pressure readings ([i] SBP <140 mm Hg and DBP <90 mm Hg; [ii] SBP ≥140‐159 mm Hg or DBP ≥90‐99 mm Hg; [iii] SBP ≥160‐179 mm Hg or DBP ≥100‐109 mm Hg; [iv] SBP ≥180 mm Hg or DBP ≥110 mm Hg) was assessed in terms of the percentage of patients who experienced “no shift”, “categorical increase”, or “categorical decrease” from baseline to EoT. The percentage of patients meeting change from baseline criteria for SBP (≥10, 15, 20 mm Hg), DBP (≥5, 10, 15 mm Hg), and pulse rate (≥5, 10, 15 bpm) at three consecutive postbaseline visits was presented in the overall population and in the subpopulations based on hypertensive status at screening or β‐blocker use. The percentage of patients in the overall population who experienced potentially clinically significant changes in vital signs (SBP [≥180 mm Hg and a ≥20 mm Hg change from baseline]; DBP [≥105 mm Hg and ≥15 mm Hg change from baseline]; pulse rate [≥120 bpm and ≥15 bpm change from baseline]) was also reported.
Twelve‐lead electrocardiograms (ECGs) were performed at screening, baseline (week 0) and at each follow‐up visit with the patient in the supine position, after the patient had been lying down for 15 minutes. Triplicate ECGs were recorded with an interval of at least 5 minutes between each ECG, made at a speed of 25 mm/s and all leads including at least 4 complexes. Interpretation of the ECG was undertaken by the investigator (not reported in this analysis) and by an independent central reader. The percentage of patients with abnormal/normal readings were reported at each visit. The mean values and change from baseline at EoT in the PR interval, RR interval, QRS interval, QT interval corrected by Fridericia's formula (QTcF), and heart rate are reported in the overall population. The frequency of QTcF extreme values (>450 mseconds, >480 mseconds and >500 mseconds) and extremes in change from baseline (>30 mseconds and >60 mseconds) were reported during double‐blind treatment in the overall population and stratified by gender. For the interpretation of the ECGs by the central reader, the percentage of patients who had “no categorical change”, “abnormal to normal” and “normal to abnormal” during double‐blind treatment were presented.
2.3. Statistical analysis
Sample size calculations related to categorical changes in the primary efficacy endpoint (incontinence episodes/24 h), were based on previous studies with mirabegron alone and in combination with solifenacin, and are described in the primary analysis of BESIDE.25 The safety analysis set (SAF) consisted of all randomised patients who received ≥1 dose of double‐blind treatment and was used for the analysis of CV safety variables.
CV TEAEs of interest (increased blood pressure; QT prolongation; increased heart rate, tachycardia, atrial fibrillation and palpitations) were identified using standardised MedRA queries (v16.0). The percentage of patients who reported ≥1 AE were summarised by System Organ Class and Preferred Term by treatment with corresponding 95% CI. Tachycardia was reported as a TEAE according to the investigator's judgement independent of a pulse rate ≥100 bpm. Tachycardia was also reported according to the standardised assessment of vital sign data based on a mean pulse rate ≥100 bpm.
The average change from baseline to EoT for vital signs was analysed using an analysis of covariance (ANCOVA) model which included treatment group, randomisation stratification factors (gender, age group <65 and ≥65 years, geographic region and 4‐week incontinence episode reduction group) as fixed factors and baseline vital sign value as a covariate. Least squares mean estimates and two‐sided 95% CIs for the mean changes from baseline within each treatment group, as well as for the difference in change from baseline between combination therapy vs. solifenacin 5 mg and 10 mg, were derived with two‐sided 95% CIs. The study was only powered for efficacy endpoints, thus no P‐values were calculated for vital sign differences. Populations for vital sign analyses included: (i) overall (any patient in the SAF); (ii) past history of hypertension (medical history of hypertension and no concurrent antihypertensive treatment at screening); (iii) hypertensive at screening (medical history of hypertension and concurrent antihypertensive treatment at screening); (iv) no hypertension at screening; (v) use of β‐blockers (≥1 dose during the run‐in period and ≥1 dose during double‐blind treatment); (vi) no use of β‐blockers. For patients meeting change from baseline criteria in vital signs, percentages are based on the total number in each treatment group with non‐missing values. Additional sensitivity analyses were performed including antihypertensive use at screening or a different age group factor (<45, ≥45 to <65, ≥65 years) in the ANCOVA model to analyse changes from baseline in vital signs.
Heart rate, PR interval, RR interval, QRS interval and QTcF were summarised descriptively for each treatment group at each visit including changes from baseline to each postbaseline visit. The worst non‐missing value was used in the analysis for categorical ECG variables reported by the central reader.
3. Results
3.1. Patient demographics and baseline characteristics
Overall, 2174 patients were randomised into the study, of which 2172 (99.9%) received double‐blind study drug and were included in the safety analysis set (SAF). The discontinuation rate (post‐randomisation) was low (6.2%) with no relevant differences among treatment groups. The overall mean age at screening was 57.5 years. Approximately 37% of patients had a history of hypertension and approximately 7.5% and 4.5%, respectively, had type 1 and type 2 diabetes mellitus in each treatment group at baseline (Table 1). The most frequently reported concomitant medications during treatment were agents acting on the renin‐angiotensin system (~27%); medication use was comparable across treatment groups (Table 1).
Table 1.
Patient demographics and CV‐related baseline characteristics (SAF)
| Combination n=725 | Solifenacin 5 mg n=728 | Solifenacin 10 mg n=719 | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 603 (83.2) | 604 (83.0) | 600 (83.4) |
| Male | 122 (16.8) | 124 (17.0) | 119 (16.6) |
| Race, n (%) | |||
| White | 688 (94.9) | 679 (93.3) | 680 (94.6) |
| Black/African American | 20 (2.8) | 24 (3.3) | 27 (3.8) |
| Asian | 13 (1.8) | 21 (2.9) | 10 (1.4) |
| Other | 4 (0.6) | 4 (0.5) | 2 (0.3) |
| Mean (SD) age, year | 58.2 (13.1) | 56.9 (13.5) | 57.3 (13.3) |
| ≥65 year, n (%) | 231 (31.9) | 226 (31.0) | 224 (31.2) |
| ≥75 year, n (%) | 73 (10.1) | 66 (9.1) | 55 (7.6) |
| BMI (kg/m2) | |||
| Mean (SD) | 28.9 (5.9) | 29.1 (6.2) | 28.9 (6.0) |
| Previous OAB medication (prior to screening), n (%) | 485 (66.9) | 503 (69.1) | 491 (68.3) |
| CV history (system organ class, preferred term) affecting ≥1% of patients in any treatment group, n (%) | |||
| Vascular disorders | 308 (42.5) | 294 (40.4) | 283 (39.4) |
| Hypertension | 270 (37.2) | 262 (36.0) | 262 (36.4) |
| Varicose vein | 35 (4.8) | 21 (2.9) | 19 (2.6) |
| Essential hypertension | 2 (0.3) | 10 (1.4) | 3 (0.4) |
| Venous insufficiency | 4 (0.6) | 7 (1.0) | 4 (0.6) |
| Cardiac disorders | 62 (8.6) | 59 (8.1) | 61 (8.5) |
| Myocardial ischaemia | 14 (1.9) | 12 (1.6) | 14 (1.9) |
| Coronary artery disease | 14 (1.9) | 15 (2.1) | 6 (0.8) |
| Myocardial infarction | 7 (1.0) | 5 (0.7) | 9 (1.3) |
| Arrhythmia | 7 (1.0) | 2 (0.3) | 5 (0.7) |
| Metabolism and nutrition disorders | 191 (26.3) | 211 (29.0) | 199 (27.7) |
| Hypercholesterolaemia | 67 (9.2) | 95 (13.0) | 74 (10.3) |
| Diabetes mellitus | 45 (6.2) | 66 (9.1) | 51 (7.1) |
| Obesity | 43 (5.9) | 37 (5.1) | 37 (5.1) |
| Type 2 diabetes mellitus | 23 (3.2) | 43 (5.9) | 30 (4.2) |
| Hyperlipidaemia | 23 (3.2) | 26 (3.6) | 35 (4.9) |
| Dyslipidaemia | 12 (1.7) | 9 (1.2) | 13 (1.8) |
| Vitamin D deficiency | 8 (1.1) | 6 (0.8) | 10 (1.4) |
| Gout | 8 (1.1) | 5 (0.7) | 5 (0.7) |
| Hyperuricaemia | 2 (0.3) | 7 (1.0) | 5 (0.7) |
| CV‐related concomitant medication use during double blind treatment period, n (%) | |||
| Antihypertensivesa | 22 (3.0) | 17 (2.3) | 9 (1.3) |
| β‐blocking agents | 90 (12.4) | 95 (13.0) | 101 (14.0) |
| Calcium channel blockers | 53 (7.3) | 49 (6.7) | 48 (6.7) |
| Agents acting on renin‐angiotensin system | 205 (28.3) | 196 (26.9) | 194 (27.0) |
| Lipid‐modifying agents | 119 (16.4) | 152 (20.9) | 130 (18.1) |
| Antithrombotic agents | 97 (13.4) | 95 (13.0) | 81 (11.3) |
| Drugs used in diabetes | 62 (8.6) | 95 (13.0) | 75 (10.4) |
| Cardiac therapy | 57 (7.9) | 74 (10.2) | 51 (7.1) |
| Vital signs, mean (SD) | n=724 | n=728 | n=719 |
| SBP mm Hg | 126.73 (13.83) | 125.63 (14.24) | 125.88 (14.64) |
| DBP mm Hg | 76.43 (8.31) | 76.15 (8.63) | 75.70 (8.41) |
| Pulse rate bpm | 71.35 (9.62) | 71.33 (9.47) | 71.17 (9.20) [n=718] |
| ECG parameters (Central reader) n (%) | n=721 | n=727 | n=719 |
| QTcF >450 mseconds | 31 (4.3) | 24 (3.3) | 17 (2.4) |
| QTcF >480 mseconds | 1 (0.1) | 1 (0.1) | 0 |
| QTcF >500 mseconds | 0 | 0 | 0 |
BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; ECG, electrocardiogram; OAB, overactive bladder; QTcF, QT interval corrected using Fridericia's formula; SBP, systolic blood pressure; SE, standard error.
Alpha adrenoceptor antagonists, imidazoline receptor agonists, tadalafil, magnesium sulphate, pyrimidine derivatives, hydrazinophthalazine derivatives, methyldopa, rauwolfia alkaloids.
3.2. CV‐related TEAEs
Overall, the frequency of TEAEs of interest related to the CV system (increased blood pressure; QT prolongation; increased heart rate, tachycardia, atrial fibrillation and palpitations) was low (<2.0%) and comparable across treatment groups. Hypertension as a TEAE was reported in 19 patients (combination n=8, [1.1%], solifenacin 5 mg, n=5 [0.7%], solifenacin 10 mg, n=6 [0.8%]) (Table 2). One patient (0.1%) in each group had ECG QT prolongation (the three patients were female with QTcF readings that ranged from 447 mseconds to 476 mseconds). Tachycardia was reported as a TEAE in three patients (combination, n=2 [0.3%]; solifenacin 5 mg, n=1 [0.1%]); ECG and vital sign results confirmed heart rate was no more than 102 bpm in these patients. Seven serious CV‐related TEAEs were reported: acute myocardial infarction (n=1 [0.1%], solifenacin 10 mg), atrial fibrillation (n=1 [0.1%], solifenacin 5 mg), atrioventricular block complete (n=1 [0.1%], combination) arteriogram coronary normal (n=1 [0.1%], combination), transient ischaemic attack (n=1 [0.1%], solifenacin 10 mg), hypertensive crisis (n=1 [0.1%], solifenacin 10 mg) and thrombosis (n=1, solifenacin 5 mg). The patient in the solifenacin 10 mg group who experienced hypertensive crisis required hospitalisation for 1 day and temporary cessation of solifenacin treatment. The patient experiencing atrioventricular block in the combination group had a cardiac history (ie, previous cardiac surgery, myocardial infarction, and aortic aneurysm) and second degree block prior to double‐blind treatment. The case of thrombosis developed in a previous femoral bypass. The coronary arteriogram was requested after an episode of pneumonia that was considered clinically significant by the study investigator. None of the seven serious TEAEs were considered by the investigator to be related to treatment. Ten potentially serious CV‐related TEAEs were reviewed by the adjudication committee. This included five non‐APTC/MACE CV events (combination n=1 [0.1%], solifenacin 5 mg n=2 [0.3%] and solifenacin 10 mg n=2 [0.3%]) and one APTC/MACE CV event (non‐fatal myocardial infarction) in the solifenacin 10 mg group. In addition, there was one event in the solifenacin 10 mg group for which there was insufficient data to adjudicate, and three events in the combination group were adjudicated as non‐CV events.
Table 2.
Treatment‐emergent adverse events (TEAEs) (MedRA v16.0) of interest related to the CV system, serious CV‐related TEAEs and frequency of adjudicated CV‐related TEAEs (SAF)
| TEAEs of interest SOC and PT | Patients, n (%) [95% CI] | ||
|---|---|---|---|
| Combination (n=725) | Solifenacin 5 mg (n=728) | Solifenacin 10 mg (n=719) | |
| Increased blood pressure | |||
| Overall | 12 (1.7) [0.7 to 2.6] | 6 (0.8) [0.2 to 1.5] | 13 (1.8) [0.8 to 2.8] |
| Vascular disorders | 8 (1.1) [0.3 to 1.9] | 5 (0.7) [0.1 to 1.3] | 7 (1.0) [0.3 to 1.7] |
| Hypertension | 8 (1.1) [0.3 to 1.9] | 5 (0.7) [0.1 to 1.3] | 6 (0.8) [0.2 to 1.5] |
| Hypertensive crisis | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Investigations | 4 (0.6) [0.0 to 1.1] | 1 (0.1) [0.0 to 0.4] | 6 (0.8) [0.2 to 1.5] |
| Blood pressure increased | 4 (0.6) [0.0 to 1.1] | 1 (0.1) [0.0 to 0.4] | 6 (0.8) [0.2 to 1.5] |
| QT prolongation | |||
| Overall | 1 (0.1) [0.0 to 0.4] | 1 (0.1) [0.0 to 0.4] | 2 (0.3) [0.0 to 0.7] |
| Investigations | 1 (0.1) [0.0 to 0.4] | 1 (0.1) [0.0 to 0.4] | 1 (0.1) [0.0 to 0.4] |
| ECG QT prolonged | 1 (0.1) [0.0 to 0.4] | 1 (0.1) [0.0 to 0.4] | 1 (0.1) [0.0 to 0.4] |
| Nervous system disorders | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Syncope | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Increased heart rate, tachycardia, atrial fibrillation and palpitations | |||
| Overall | 7 (1.0) [0.3 to 1.7] | 5 (0.7) [0.1 to 1.3] | 4 (0.6) [0.0 to 1.1] |
| Cardiac disorders | 7a (1.0) [0.3 to 1.7] | 5 (0.7) [0.1 to 1.3] | 3 (0.4) [0.0 to 0.9] |
| Palpitations | 6 (0.8) [0.2 to 1.5] | 2 (0.3) [0.0 to 0.7] | 2 (0.3) [0.0 to 0.7] |
| Tachycardia | 2 (0.3) [0.0 to 0.7] | 1 (0.1) [0.0 to 0.4] | 0 |
| Atrial fibrillation | 0 | 1 (0.1) [0.0 to 0.4] | 0 |
| Supraventricular extrasystoles | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Ventricular extrasystoles | 0 | 1 (0.1) [0.0 to 0.4] | 0 |
| Nervous system disorders | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Syncope | 0 | 0 | 1 (0.1) [0.0 to 0.4] |
| Serious CV‐related TEAEs (PT only) | |||
| Arteriogram coronary normal | 1 (0.1) | 0 | 0 |
| Acute myocardial infarction | 0 | 0 | 1 (0.1) |
| Atrial fibrillation | 0 | 1 (0.1) | 0 |
| Atrioventricular block complete | 1 (0.1) | 0 | 0 |
| Transient ischaemic attack | 0 | 0 | 1 (0.1) |
| Hypertensive crisis | 0 | 0 | 1 (0.1) |
| Thrombosis | 0 | 1 (0.1) | 0 |
| Adjudicated serious CV‐related TEAEs | |||
| Overall | 4 (0.6) | 2 (0.3) | 4 (0.6) |
| APTC/MACE CV events | 0 | 0 | 1 (0.1) |
| Non‐fatal myocardial infarction | 0 | 0 | 1 (0.1) |
| Non‐fatal stroke | 0 | 0 | 0 |
| CV death | 0 | 0 | 0 |
| Non‐APTC/MACE CV events | 1 (0.1) | 2 (0.3) | 2 (0.3) |
| Unstable angina | 0 | 0 | 0 |
| Coronary revascularization | 0 | 0 | 0 |
| Transient ischaemic attack | 0 | 0 | 1 (0.1) |
| Venous and peripheral arterial vascular thrombotic event | 0 | 1 (0.1) | 0 |
| Congestive heart failure | 0 | 0 | 0 |
| Arrhythmia, no evidence of ischaemia | 1 (0.1) | 1 (0.1) | 0 |
| Other serious non‐MACE CV event | 0 | 0 | 1 (0.1) |
| Other | 3 (0.4) | 0 | 1 (0.1) |
| Insufficient data | 0 | 0 | 1 (0.1) |
| Non‐CV event | 3 (0.4) | 0 | 0 |
APTC, antiplatelet trialists collaboration; CV, cardiovascular; ECG, electrocardiogram; MACE, major adverse cardiovascular event; PT, preferred term; SOC, system organ class; TEAEs, treatment‐emergent adverse events.
TEAE refers to an AE which started or worsened in the period from first double‐blind medication intake until 30 days after the last double‐blind medication intake.
One patient experienced both tachycardia and palpitations; the patient is counted once under each of palpitations and tachycardia, but only once under the SOC cardiac disorders.
3.3. Vital signs
In the overall SAF population, the adjusted mean (SE) change from baseline to EoT in SBP was 0.07 (0.38), −0.93 (0.38) and −1.28 (0.38) for combination, solifenacin 5 mg and solifenacin 10 mg groups, respectively; resulting in a mean difference of ~1 mm Hg in the combination group vs. solifenacin monotherapy. There were no appreciable differences between combination and solifenacin monotherapy for DBP and pulse rate: the adjusted mean (SE) change from baseline to EoT in DBP was −0.35 (0.26), −0.45 (0.26) and −0.48 (0.26) and in pulse rate was 0.47 (0.28), 0.43 (0.28) and 0.27 (0.28) in the combination, solifenacin 5 mg and solifenacin 10 mg groups, respectively. There were no notable differences between combination and solifenacin monotherapies in vital signs in subpopulations stratified by hypertensive status and β‐blocker use, in the sensitivity analyses using a different age group factor or hypertensive medication use at screening in the model (Figure 1), and between male and female patients (Table 3).
Figure 1.
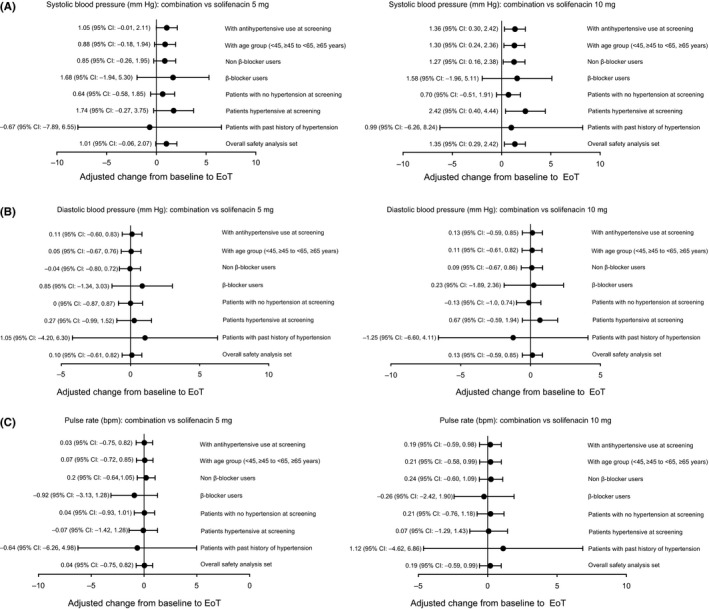
Adjusted change from baseline to EoT for vital signs in the overall population and by sensitivity analyses: A, systolic blood pressure: combination vs. solifenacin 5 mg and 10 mg; B, diastolic blood pressure: combination vs. solifenacin 5 mg and 10 mg; C, Pulse rate: combination vs. solifenacin 5 mg and 10 mg
Table 3.
Adjusted change from baseline to EoT in vital signs by sex
| Mean (SE) [95% CI] | |||
|---|---|---|---|
| Combination | Solifenacin 5 mg | Solifenacin 10 mg | |
| Males | n=119 | n=120 | n=114 |
| Systolic blood pressure | |||
| Adjusted change from baseline | 1.82 (0.94) [−0.03 to 3.67] | 1.46 (0.93) [−0.37 to 3.29] | 0.89 (0.96) [−0.99 to 2.78] |
| Difference vs. solifenacin 5 mg | 0.36 (1.32) [−2.23 to 2.95] | ||
| Difference vs. solifenacin 10 mg | 0.92 (1.34) [−1.70 to 3.55] | ||
| Diastolic blood pressure | |||
| Adjusted change from baseline | 1.37 (0.63) [0.14 to 2.61] | 1.10 (0.63) [−0.13 to 2.34] | 0.43 (0.64) [−0.83 to 1.70] |
| Difference vs. solifenacin 5 mg | 0.27 (0.89) [−1.47 to 2.01] | ||
| Difference vs. solifenacin 10 mg | 0.94 (0.90) [−0.82 to 2.71] | ||
| Pulse rate | |||
| Adjusted change from baseline | 0.28 (0.69) [−1.07 to 1.64] | 1.41 (0.69) [0.06 to 2.76] | 0.26 (0.71) [−1.13 to 1.65] |
| Difference vs. solifenacin 5 mg | −1.13 (0.97) [−3.04 to 0.79] | ||
| Difference vs. solifenacin 10 mg | 0.02 (0.99) [−1.91 to 1.96] | ||
| Females | n=587 | n=591 | n=592 |
| Systolic blood pressure | |||
| Adjusted change from baseline | −0.27 (0.42) [−1.10 to 0.55] | −1.41 (0.42) [−2.24 to −0.59] | −1.71 (0.42) [−2.53 to −0.89] |
| Difference vs. solifenacin 5 mg | 1.14 (0.59) [−0.03 to 2.31] | ||
| Difference vs. solifenacin 10 mg | 1.44 (0.59) [0.27 to 2.60] | ||
| Diastolic blood pressure | |||
| Adjusted change from baseline | −0.69 (0.28) [−1.25 to −0.14] | −0.76 (0.28) [−1.32 to −0.21] | −0.66 (0.28) [−1.22 to −0.11] |
| Difference vs. solifenacin 5 mg | 0.07 (0.40) [−0.71 to 0.85] | ||
| Difference vs. solifenacin 10 mg | −0.03 (0.40) [−0.81 to 0.75] | ||
| Pulse rate | |||
| Adjusted change from baseline | 0.50 (0.31) [−0.11 to 1.11] | 0.23 (0.31) [−0.38 to 0.84] | 0.27 (0.31) [−0.33 to 0.88] |
| Difference vs. solifenacin 5 mg | 0.27 (0.44) [−0.59 to 1.13] | ||
| Difference vs. solifenacin 10 mg | 0.23 (0.44) [−0.63 to 1.09] | ||
Adjusted change from baseline values and 95% CIs are generated from ANCOVA model with treatment group, gender, age group, 4‐week incontinence reduction group, geographic region and interaction between age group (<65, ≥65 years) and treatment group as fixed factors and baseline as a covariate.
Differences of adjusted means are calculated by subtracting adjusted mean of solifenacin treatment from adjusted mean of combination treatment.
The percentage of patients with increases in vital signs at EoT that met the change from baseline criteria at three consecutive visits was low and similar across treatment groups in the overall population (Figure 2A‐C). Similar findings were evident in the subpopulations according to hypertension history, hypertensive status at screening and β‐blocker use, with the exception of pulse rate in the past history of hypertension cohort (n=53) in which no more than 3/17 (17.6%) patients receiving combination met one of the three criteria for change from baseline vs. none in the solifenacin groups (Table 4).
Figure 2.
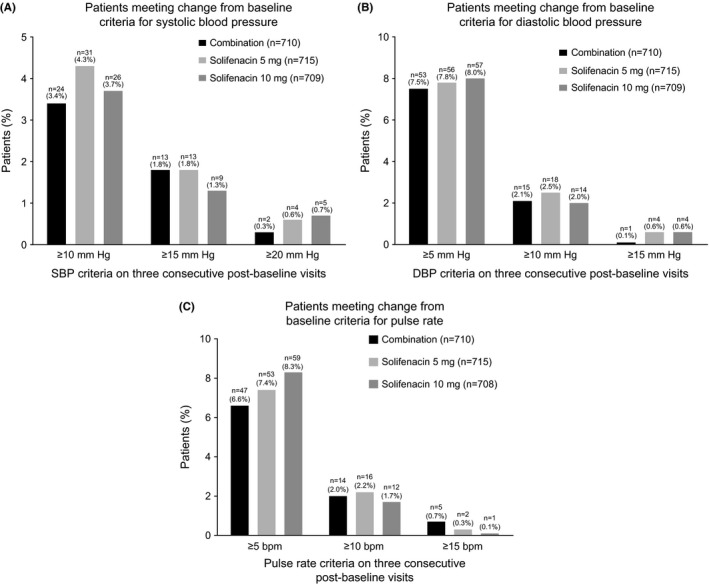
A‐C, Patients meeting change from baseline criteria for systolic blood pressure (A), diastolic blood pressure (B) and pulse rate (C)
Table 4.
Patients meeting change from baseline criteria in vital signs by subpopulation (based on patients with three consecutive postbaseline values): past history of hypertension; hypertensive at screening; no hypertension at screening; β‐blocker user; non β‐blocker user
| Combination | Solifenacin 5 mg | Solifenacin 10 mg | |
|---|---|---|---|
| Past history of hypertension | n=17 | n=18 | n=17 |
| SBP: increase from baseline | |||
| ≥10 mm Hg | 0 | 2 (11.1%) | 0 |
| ≥15 mm Hg | 0 | 0 | 0 |
| ≥20 mm Hg | 0 | 0 | 0 |
| DBP: increase from baseline | |||
| ≥5 mm Hg | 0 | 0 | 2 (11.8%) |
| ≥10 mm Hg | 0 | 0 | 1 (5.9%) |
| ≥15 mm Hg | 0 | 0 | 0 |
| Pulse rate: increase from baseline | |||
| ≥5 bpm | 3 (17.6%) | 0 | 0 |
| ≥10 bpm | 2 (11.8%) | 0 | 0 |
| ≥15 bpm | 1 (5.9%) | 0 | 0 |
| Hypertensive at screening | n=255 | n=254 | n=248 |
| SBP: increase from baseline | |||
| ≥10 mm Hg | 11 (4.3%) | 10 (3.9%) | 10 (4.0%) |
| ≥15 mm Hg | 4 (1.6%) | 4 (1.6%) | 6 (2.4%) |
| ≥20 mm Hg | 2 (0.8%) | 1 (0.4%) | 3 (1.2%) |
| DBP: increase from baseline | |||
| ≥5 mm Hg | 22 (8.6%) | 23 (9.1%) | 21 (8.5%) |
| ≥10 mm Hg | 6 (2.4%) | 8 (3.1%) | 5 (2.0%) |
| ≥15 mm Hg | 0 | 2 (0.8%) | 1 (0.4%) |
| Pulse rate: increase from baseline | |||
| ≥5 bpm | 15 (5.9%) | 23 (9.1%) | 16 (6.5%) [n=247] |
| ≥10 bpm | 4 (1.6%) | 10 (3.9%) | 3 (1.2%) [n=247] |
| ≥15 bpm | 1 (0.4%) | 1 (0.4%) | 0 |
| No hypertension at screening | n=455 | n=461 | n=461 |
| SBP: increase from baseline | |||
| ≥10 mm Hg | 13 (2.9%) | 21 (4.6%) | 16 (3.5%) |
| ≥15 mm Hg | 9 (2.0%) | 9 (2.0%) | 3 (0.7%) |
| ≥20 mm Hg | 0 | 3 (0.7%) | 2 (0.4%) |
| DBP: increase from baseline | |||
| ≥5 mm Hg | 31 (6.8%) | 33 (7.2%) | 36 (7.8%) |
| ≥10 mm Hg | 9 (2.0%) | 10 (2.2%) | 9 (2.0%) |
| ≥15 mm Hg | 1 (0.2%) | 2 (0.4%) | 3 (0.7%) |
| Pulse rate: increase from baseline | |||
| ≥5 bpm | 32 (7.0%) | 30 (6.5%) | 43 (9.3%) |
| ≥10 bpm | 10 (2.2%) | 6 (1.3%) | 9 (0.2%) |
| ≥15 bpm | 4 (0.9%) | 1 (0.2%) | 1 (0.2%) |
| β‐blocker users | n=85 | n=92 | n=101 |
| SBP: increase from baseline | |||
| ≥10 mm Hg | 2 (2.4%) | 2 (2.2%) | 4 (4.0%) |
| ≥15 mm Hg | 1 (1.2%) | 2 (2.2%) | 1 (1.0%) |
| ≥20 mm Hg | 0 | 0 | 0 |
| DBP: increase from baseline | |||
| ≥5 mm Hg | 6 (7.1%) | 4 (4.3%) | 4 (4.0%) |
| ≥10 mm Hg | 2 (2.4%) | 1 (1.1%) | 1 (1.0%) |
| ≥15 mm Hg | 0 | 0 | 0 |
| Pulse rate: increase from baseline | |||
| ≥5 bpm | 4 (4.7%) | 6 (6.5%) | 10 (9.9%) |
| ≥10 bpm | 0 | 3 (3.3%) | 2 (2.0%) |
| ≥15 bpm | 0 | 1 (1.1%) | 0 |
| Non β‐blocker users | N=622 | N=622 | N=608 |
| SBP: increase from baseline | |||
| ≥10 mm Hg | 22 (3.5%) | 29 (4.7%) | 22 (3.6%) |
| ≥15 mm Hg | 12 (1.9%) | 11 (1.8%) | 8 (1.3%) |
| ≥20 mm Hg | 2 (0.3%) | 4 (0.6%) | 5 (0.8%) |
| DBP: increase from baseline | |||
| ≥5 mm Hg | 46 (7.4%) | 52 (8.4%) | 53 (8.7%) |
| ≥10 mm Hg | 13 (2.1%) | 17 (2.7%) | 13 (2.1%) |
| ≥15 mm Hg | 1 (0.2%) | 4 (0.6%) | 4 (0.7%) |
| Pulse rate: increase from baseline | |||
| ≥5 bpm | 43 (6.9%) | 47 (7.6%) | 49 (8.1%) [n=607] |
| ≥10 bpm | 14 (2.3%) | 13 (2.1%) | 10 (1.6%) [n=607] |
| ≥15 bpm | 5 (0.8%) | 1 (0.2%) | 1 (0.2%) [n=607] |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
At EoT, a similar proportion of patients (~80%) in each treatment group had no shift in vital sign severity, while less than 10% of patients in each group experienced a categorical increase (Figure 3); three patients (n=1 combination, n=1 solifenacin 5 mg, n=1 solifenacin 10 mg) shifted to severity category 4 (SBP ≥180 mm Hg and DBP=100 mm Hg) from category 2 (SBP ≥140‐159 mm Hg and DBP ≥90‐99 mm Hg).
Figure 3.
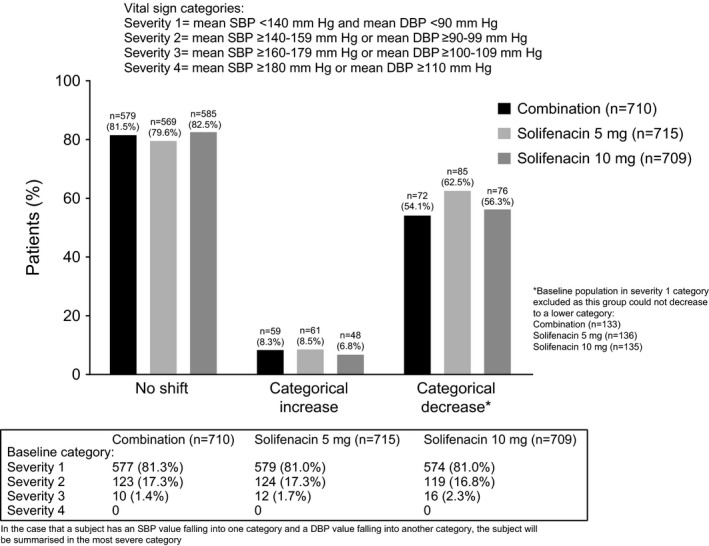
Vital sign shift at EoT
During the treatment period, potentially clinically significant values in DBP (≥105 mm Hg and ≥15 mm Hg change from baseline) were observed in six patients (n=2 [solifenacin 5 mg at week 4], n=3 [solifenacin 10 mg during the study], and n=1 [combination at week 12]), and in SBP (≥180 mm Hg and ≥20 mm Hg change from baseline) in two patients (solifenacin 10 mg group at week 4 [n=1] and 8 [n=1]).
The frequency of tachycardia, according to the standardised assessment of vital sign data, and defined as an average pulse rate ≥100 bpm, was low and similar across groups during the treatment period (combination n=8, 1.1%; solifenacin 5 mg n=12, 1.7% and solifenacin 10 mg n=10, 1.4%).
3.4. ECG results
Mean changes from baseline to EoT in ECG parameters (PR, RR, QRS, QTcF and heart rate) were generally similar across treatment groups; the most notable exception being the QTcF, which was higher in the solifenacin 10 mg (3.30 mseconds) group vs. combination (0.49 mseconds) and solifenacin 5 mg (0.77 mseconds) (Table 5). The percentage of patients with a normal (~72%‐75%) and abnormal (~25%‐28%) ECG assessment at baseline and at each visit was comparable across each treatment group. This was reflected by the observation that >80% of patients in each group had no change in ECG interpretation (Figure 4). During double‐blind treatment, there were no patients with a mean QTcF value >500 mseconds, and 11 patients had mean QTcF values >480 mseconds (combination n=4 [0.6%] and solifenacin 10 mg n=7 [1.0%]). A slightly higher proportion of patients in the solifenacin 10 mg group had mean changes from baseline of >30 mseconds and >60 mseconds in QTcF compared with combination and solifenacin 5 mg (Figure 5A). There were no notable treatment differences in QTcF values or change from baseline by gender; however, in general, females were more likely to experience a QTcF >450 mseconds and >480 mseconds, and a change from baseline >60 mseconds compared with males (Figure 5B and C).
Table 5.
12‐lead ECG results: mean values and change from baseline at EoT (assessment by central reader)
| Mean (SD) | |||
|---|---|---|---|
| Combination (n=725) | Solifenacin 5 mg (n=728) | Solifenacin 10 mg (n=719) | |
| PR duration (mseconds) | |||
| Baseline | 165.62 (23.08) [n=713] | 165.18 (24.02) [n=722] | 165.15 (22.89) [n=714] |
| EoT | 165.52 (22.91) [n=703] | 165.48 (23.57) [n=710] | 166.20 (22.49) [n=704] |
| Change from baseline | 0.08 (11.82) [n=699] | 0.48 (11.97) [n=708] | 0.95 (10.67) [n=704] |
| RR duration (mseconds) | |||
| Baseline | 894.20 (131.01) [n=721] | 891.15 (136.96) [n=727] | 892.71 (123.71) [n=719] |
| EoT | 870.00 (123.13) [n=710] | 877.50 (136.25) [n=714] | 880.70 (130.68) [n=709] |
| Change from baseline | −23.07 (102.39) [n=707] | −13.34 (106.44) [n=713] | −12.29 (102.12) [n=709] |
| QRS duration (mseconds) | |||
| Baseline | 92.92 (9.58) [n=721] | 92.74 (10.10) [n=727] | 92.46 (8.60) [n=719] |
| EoT | 93.71 (9.88) [n=710] | 93.48 (9.81) [n=714] | 93.47 (8.82) [n=709] |
| Change from baseline | 0.82 (5.19) [n=707] | 0.73 (6.10) [n=713] | 0.98 (6.23) [n=709] |
| QTcF (mseconds) | |||
| Baseline | 417.34 (18.00) [n=721] | 416.52 (18.09) [n=727] | 415.42 (17.85) [n=719] |
| EoT | 417.85 (18.99) [n=710] | 417.35 (17.67) [n=714] | 418.77 (19.01) [n=709] |
| Change from baseline | 0.49 (13.23) [n=707] | 0.77 (12.98) [n=713] | 3.30 (13.72) [n=709] |
| Heart rate (bpm) | |||
| Baseline | 68.61 (9.90) [n=721] | 69.03 (10.53) [n=727] | 68.63 (9.73) [n=719] |
| EoT | 70.41 (9.77) [n=710] | 70.13 (10.84) [n=714] | 69.73 (10.43) [n=709] |
| Change from baseline | 1.71 (8.19) [n=707] | 1.08 (8.65) [n=713] | 1.12 (8.22) [n=709] |
EoT, end of treatment; QTcF, QT interval corrected using Fridericia's formula.
Figure 4.
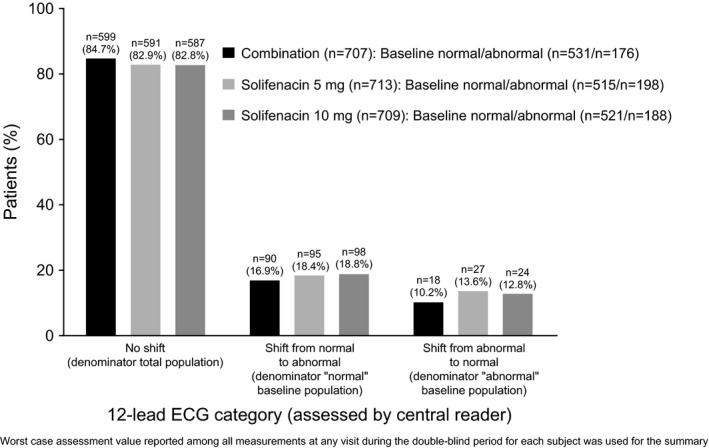
Shift categories for interpretation of 12‐lead electrocardiogram results during double‐blind treatment (assessment by central reader)
Figure 5.
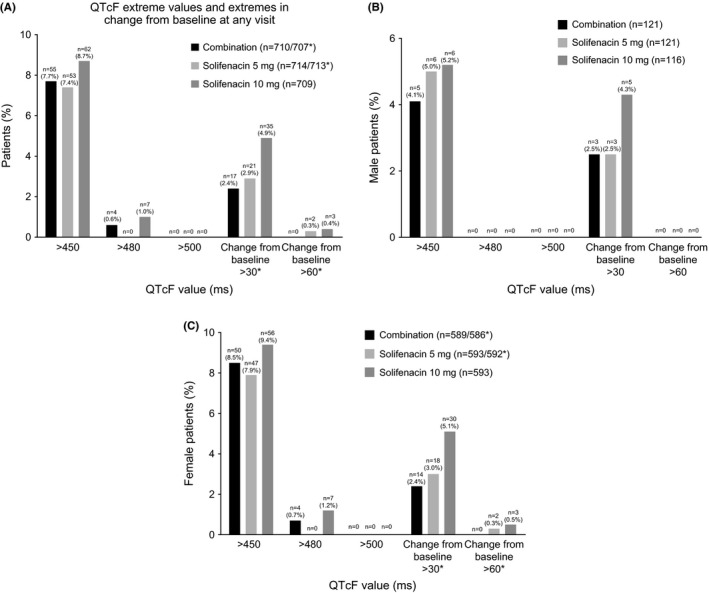
Frequency of QT interval corrected using Fridericia's formula extreme values and extremes in change from baseline at any visit in the overall population (A), male (B) and female patients (C); results are based on the worst postbaseline mean value during the double‐blind treatment period
4. Discussion
CV safety concerns are particularly relevant in chronic medical conditions such as OAB, in which patients have a significantly higher rate of pre‐existing CV comorbidities than patients without OAB.8 Of the two oral pharmacotherapies investigated in BESIDE, solifenacin selectively inhibits the M3 receptor subtype in the parasympathetic system, and mirabegron selectively stimulates the β3 adrenoceptor in the sympathetic system, hence the potential for additional systemic effects beyond the bladder, including the heart and vasculature. OAB studies investigating mirabegron monotherapy (25 mg/d or 50 mg/d) for 12 weeks and 12 months, or solifenacin (5 mg/d or 10 mg/d) monotherapy for 12 weeks have not demonstrated an increased CV risk, with no clinically relevant changes in cardiac function or blood pressure reported.18, 19 However, the concomitant exposure to these agents with distinct mechanisms of action raises the potential for a synergistic effect on the CV system.
This assessment from the BESIDE study is the first to investigate CV safety in OAB patients treated with a combination of mirabegron and solifenacin. The OAB population represents a real‐life clinical setting characterised by refractory, treatment‐experienced patients with a moderate level of CV risk (ie, hypertension, diabetes) and polypharmacy; almost one‐third of the population were controlled hypertensive patients. In this analysis of CV safety, the frequency of CV TEAEs, and change in vital signs and ECG results, were comparable between combination therapy and solifenacin monotherapy (5 mg or 10 mg) confirming no synergistic effect on the CV system regardless of pre‐existing CV risk factors (eg, hypertension history or hypertensive at screening).
The frequency of CV TEAEs and the magnitude of change in vital signs and ECG parameters with combination therapy is consistent with mirabegron and solifenacin monotherapy,18, 19 and with other studies that have investigated a combination of mirabegron and solifenacin in a Japanese OAB population26 and in a Phase II dose‐ranging study.27 The frequency of hypertension (<2%) across treatment groups in BESIDE is considerably lower than that reported with placebo and mirabegron monotherapy in previous 12‐week Phase III studies (placebo n=7.6%, mirabegron 50 mg n=7.5%).29 However, hypertension was defined using three prespecified criteria in the previous Phase III monotherapy trials, whereas in BESIDE there were no predefined criteria and hypertension was reported as an AE, if considered clinically relevant by the investigator. Similarly, the overall frequency of tachycardia (reported as a TEAE by the investigator or a mean pulse rate ≥100 bpm) with combination therapy (1.4%) was lower than that reported with mirabegron 50 mg monotherapy in the previous 12‐week studies (3.8%).29
The frequency of CV TEAEs reported in BESIDE compares favourably with the rate of CV events reported in the general population. In the Framingham study, after 30 years of follow‐up, the frequency of hypertension (SBP ≥160 mm Hg and DBP ≥95 mm Hg) increased with age in men from 3.3% at ages 30‐39 to 6.2% at ages 70‐79, and in women from 1.5% at ages 30‐39 to 8.6% at ages 70‐79.30 The frequency of tachycardia (≥100 bpm) in the general US population based on national reference data on resting pulse rate is 1.3% in men and 1.9% in women.31
The only notable difference in vital signs between combination and solifenacin monotherapy in BESIDE was the 1 mm Hg relative difference in SBP, which was unchanged with combination therapy and decreased with solifenacin monotherapy. The negligible effects on vital signs are reflected by the fact that only eight patients (combination n=1, solifenacin 5 mg n=2, solifenacin 10 mg n=5) had potentially significant increases in blood pressure during the study.
The frequency of ECG QT prolongation was 0.1% in each treatment group and there were no cases of extreme QTcF values >500 mseconds. Five solifenacin‐treated patients experienced a change in QTcF >60 mseconds during the study compared with none in the combination group. Regardless of treatment, female patients were more likely to experience extreme QTcF values >450 mseconds and >480 mseconds than males, which reflects the observation that corrected QT intervals are generally longer in women than in men.32
A substantial proportion of the BESIDE population was taking medication to control hypertension, which may have concealed potential increases in blood pressure; however, this would apply across all treatment arms. Furthermore, the study lacked a placebo arm, which would have allowed a more robust comparison of CV safety between active treatment and a control cohort in this refractory incontinent population. In this paper, CV safety was not reported in patients stratified by older age (≥65 years and ≥75 years)—the cohort most likely to have pre‐existing CV morbidities and at risk of developing CV‐related AEs—results for these patients will be presented in a subsequent analysis in older patients from the BESIDE study.
In addition to the safety analysis in older patients in BESIDE, future subanalyses using integrated clinical trial data could investigate combination therapy vs. placebo in other high risk CV populations (eg, diabetes, hypertension, and BMI >30), and compare office‐based vs. ambulatory blood pressure monitoring.
5. Conclusion
In OAB patients treated with a combination of mirabegron and solifenacin, the frequency of CV‐related AEs and changes from baseline in vital signs and ECG parameters were comparable with the recommended doses of solifenacin monotherapy (5 mg and 10 mg), indicating a lack of a synergistic effect on CV safety with combination. However, good clinical practice advocates regular blood pressure monitoring in older patients where CV risk may be cumulative because of comorbidities or co‐medication.
Disclosures
MJD has received research grants and consultancy/speaker fees from Astellas, Allergan and Ferring. SM has received speaker and consultancy fees from Astellas, Allergan, Medtronic and Cogentix. CRC has received grants, speaker and consultancy fees from Astellas, speaker and consultancy fees from Allergan and Boston, and consultancy fees from Medtronic, Pfizer and Recordati. AE has no financial disclosures to declare. SA has received grants and speaker fees from Astellas, and consultancy fees from Allergan. JCS has received personal fees as study investigator from Astellas and GSK. SH has received grants and consultancy fees from Astellas, Allergan and Ipsen, and consultancy fees from Pfizer, Merus and Ferring. DM has received speaker fees from Astellas. ES, MH and MS are employees of Astellas.
Author Contributions
MJD, SM, CRC, AE, SA, JCS, DM, SH, ES, MH, MS: concept/design. MJD, SM, CRC, AE, SA, JCS, DM, SH, ES, MH, MS: data analysis/interpretation. MJD, SM, CRC, AE, SA, JCS, DM, SH, ES, MH, MS: drafting article/critical revision/approval of article. MS: statistics. MJD, SM, CRC, AE, SA, JCS, DM, SH: data collection.
Acknowledgements
The study was funded by Astellas Pharma Europe Ltd, which had a role in the design and conduct of the study; management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Medical writing support (preparation of the initial and final drafts of the manuscript in collaboration with the authors) was provided by Stuart Murray, MSc (Envision Scientific Solutions), funded by Astellas Pharma Global Development.
Drake MJ, MacDiarmid S, Chapple CR, et al. Cardiovascular safety in refractory incontinent patients with overactive bladder receiving add‐on mirabegron therapy to solifenacin (BESIDE). Int J Clin Pract. 2017;71:e12944 https://doi.org/10.1111/ijcp.12944
References
- 1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21:167‐178. [DOI] [PubMed] [Google Scholar]
- 2. Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI‐RS 2013. Neurourol Urodyn. 2014;33:622‐624. [DOI] [PubMed] [Google Scholar]
- 3. Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132‐1138. [DOI] [PubMed] [Google Scholar]
- 4. Milsom I, Stewart W, Thuroff J. The prevalence of overactive bladder. Am J Manag Care. 2000;6:S565‐S573. [PubMed] [Google Scholar]
- 5. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327‐336. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal A, Eryuzlu LN, Cartwright R, et al. What is the most bothersome lower urinary tract symptom? Individual‐ and population‐level perspectives for both men and women. Eur Urol. 2014;65:1211‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sexton CC, Coyne KS, Kopp ZS, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS.. BJU Int. 2009;103(suppl 3):12‐23. [DOI] [PubMed] [Google Scholar]
- 8. Andersson KE, Sarawate C, Kahler KH, Stanley EL, Kulkarni AS. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int. 2010;106:268‐274. [DOI] [PubMed] [Google Scholar]
- 9. Andersson KE, Yoshida M. Antimuscarinics and the overactive detrusor – which is the main mechanism of action? Eur Urol. 2003;43:1‐5. [DOI] [PubMed] [Google Scholar]
- 10. Wuest M, Eichhorn B, Grimm MO, et al. Catecholamines relax detrusor through beta 2‐adrenoceptors in mouse and beta 3‐adrenoceptors in man. J Pharmacol Exp Ther. 2009;328:213‐222. [DOI] [PubMed] [Google Scholar]
- 11. Andersson KE, Campeau L, Olshansky B. Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br J Clin Pharmacol. 2011;72:186‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walch L, Brink C, Norel X. The muscarinic receptor subtypes in human blood vessels. Thérapie. 2001;56:223‐226. [PubMed] [Google Scholar]
- 13. Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651‐690. [PubMed] [Google Scholar]
- 14. Skeberdis VA, Gendviliene V, Zablockaite D, et al. Beta3‐adrenergic receptor activation increases human atrial tissue contractility and stimulates the L‐type Ca2+ current. J Clin Invest. 2008;118:3219‐3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VESICARE® (solifenacin succinate) product label. FDA.gov, Revised 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021518s008lbl.pdf. Accessed October 14, 2016.
- 16. Committee for Medicinal Products for Human Use (CHMP) . Assessment report: Betmiga. European Medicines Agency web site. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002388/WC500137308.pdf. Accessed November 10, 2016
- 17. Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65:755‐765. [DOI] [PubMed] [Google Scholar]
- 18. Rosa GM, Bauckneht M, Scala C, et al. Cardiovascular effects of antimuscarinic agents in overactive bladder. Expert Opin Drug Saf. 2013;12:815‐827. [DOI] [PubMed] [Google Scholar]
- 19. Rosa GM, Ferrero S, Nitti V, et al. Cardiovascular safety of β3‐adrenoceptor agonists for the treatment of patients with overactive bladder syndrome. Eur Urol. 2016;69:311‐323. [DOI] [PubMed] [Google Scholar]
- 20. Astellas Pharma, Inc. Prescribing information for MYRBETRIQ® (mirabegron) extended release tablets, for oral use . Revised 2015. http://www.us.astellas.com/docs/Myrbetriq_WPI.pdf. Accessed September 1, 2015.
- 21. Sexton CC, Notte SM, Maroulis C, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65:567‐585. [DOI] [PubMed] [Google Scholar]
- 22. Chapple CR, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta‐analysis. Eur Urol. 2008;54:543‐562. [DOI] [PubMed] [Google Scholar]
- 23. Chancellor MB, Migliaccio‐Walle K, Bramley TJ, et al. Long‐term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin Ther. 2013;35:1744‐1751. [DOI] [PubMed] [Google Scholar]
- 24. Benner JS, Nichol MB, Rovner ES, et al. Patient‐reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276‐1282. [DOI] [PubMed] [Google Scholar]
- 25. Drake MJ, Chapple C, Esen AA, et al. Efficacy and safety of mirabegron add‐on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4‐week solifenacin monotherapy: a randomised double‐blind multicentre phase 3B study (BESIDE). Eur Urol. 2016;70:136‐145. [DOI] [PubMed] [Google Scholar]
- 26. Yamaguchi O, Kakizaki H, Homma Y, et al. Safety and efficacy of mirabegron as ‘add‐on’ therapy in patients with overactive bladder treated with solifenacin: a post‐marketing, open‐label study in Japan (MILAI study). BJU Int. 2015;116:612‐622. [DOI] [PubMed] [Google Scholar]
- 27. Abrams P, Kelleher C, Staskin D, et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double‐blind, dose‐ranging, phase 2 study (Symphony). Eur Urol. 2015;67:577‐588. [DOI] [PubMed] [Google Scholar]
- 28. MacDiarmid S, Al‐Shukri S, Barkin J, et al. Mirabegron as add‐on treatment to solifenacin in patients with incontinent overactive bladder and an inadequate response to solifenacin monotherapy. J Urol. 2016;196:809‐818. [DOI] [PubMed] [Google Scholar]
- 29. Nitti V, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double‐blind, placebo‐controlled, phase III studies. Int J Clin Pract. 2013;67:619‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dannenberg AL, Garrison RJ, Kannel WB. Incidence of hypertension in the Framingham Study. Am J Public Health. 1988;78:676‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ostchega Y, Porter KS, Hughes J, Dillon CF, Nwankwo T. Resting pulse rate reference data for children, adolescents and adults: United States 1999‐2008. Natl Health Stat Report. 2011;41:1‐16. [PubMed] [Google Scholar]
- 32. Wolbrette D, Naccarelli G, Curtis A, et al. Gender differences in arrhythmias. Clin Cardiol. 2002;25:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]


