Figure 3.
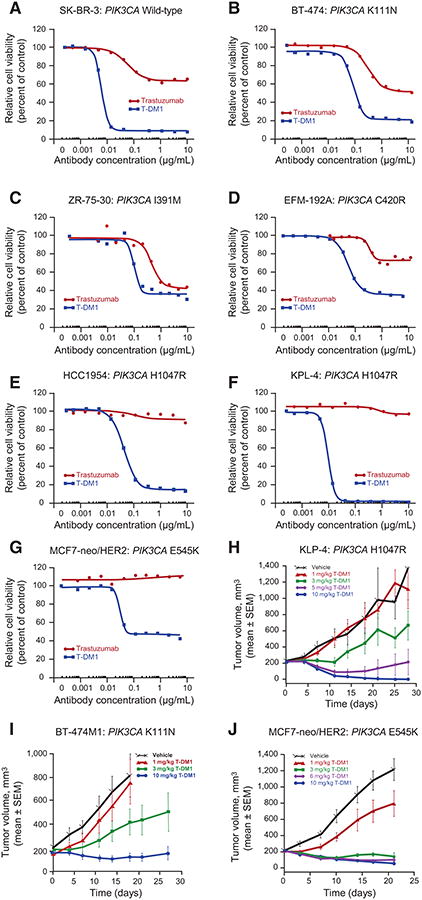
Response to T-DM1 in HER2-amplified breast cancer cell lines and xenograft models expressing mutant PIK3CA. Breast cancer cells were treated for 5 days with different concentrations of T-DM1 or trastuzumab. Cell viability was assessed using the CellTiter-Glo Luminescent Cell Viability Assay. The following cell lines were tested: SK-BR-3 (wild-type PIK3CA; A); BT-474 (mutant PIK3CA K111N, weakly activating; B); ZR-75-30 (mutant PIK3CA I391M; C); EFM-192A (mutant PIK3CA C420R, strongly activating; D); HCC1954 (mutant hotspot PIK3CA H1047R; E); KPL-4 (mutant H1047R; F); MCF7-neo/HER2 (mutant hotspot E545K; G). Antitumor efficacy of T-DM1 was assessed in PIK3CA-mutant breast cancer cell xenograft models. Xenograft models were initiated by inoculating KPL-4 cells (activating H1047R PIK3CA mutation; H) into the mammary fat pads of SCID beige mice or by inoculating BT-474M1 (nonactivating K111N PIK3CA mutation; I), or MCF7-neo/HER2 (activating E545K PIK3CA mutation; J) cells into the mammary fat pads of NCr nude mice, which had 0.36-mg 17β-estradiol 60-day sustained release pellets implanted 1 to 3 days prior to cell inoculation. In all models, once tumor volumes reached an average of 200 mm3, mice were administered a single intravenous injection of T-DM1 (doses of 1, 3, 5, 6, or 10 mg/kg) or vehicle. Tumor growth was monitored by caliper measurement.
