Figure 8.
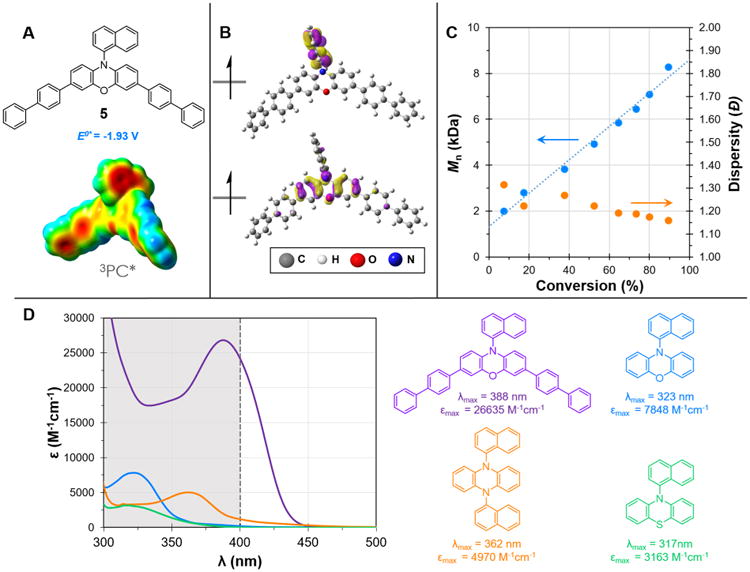
Properties of PC 5. (A) Structure, computed triplet excited state reduction potential, and ESP mapped electron density of 3PC* 5. (B) Computed triplet state SOMOs of PC 5. (C) Plot of Mn and Đ as a function of monomer conversion for the polymerization of MMA by PC 5; [MMA]:[DBMM]:[5] = [1000]:[10]:[1]; 9.35 μmol PC, 1.00 mL dimethylacetamide, and irradiated with white LEDs. (D) UV–vis spectrum of PC 5 and 1-naphthalene functionalized phenoxazine, dihydrophenazine, and phenothiazine, with color coded structures, and extinction coefficients at their respective λmax with the visible absorbance spectrum highlighted in white.
