Figure 2. DNA-PK-mediated phosphorylation of HSP90α triggers release of clients.
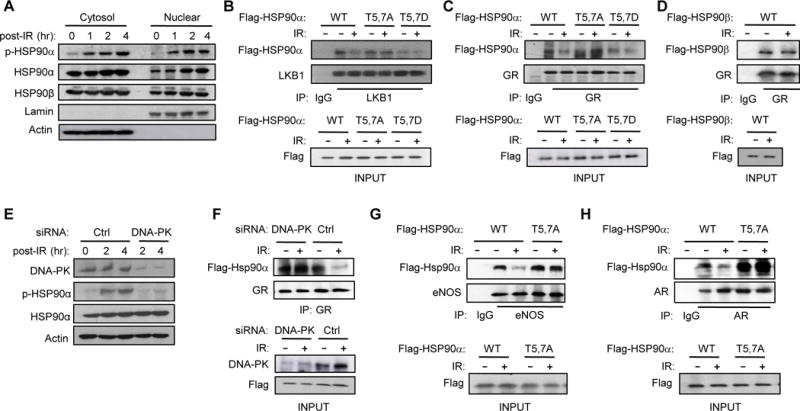
(A) T5,7 are phosphorylated in both cytoplasmic and nuclear HSP90α in response to DNA DSBs induced by IR (15 Gy) in C2C12 myotubes. (B) C2C12 myotubes were transfected with vectors encoding Flag-tagged WT or mutant HSP90α. Interaction between mutant HSP90α and endogenous LKB1 was visualized by immunoprecipitating with anti-LKB1 antibody one hr after IR and immunoblotting with anti-Flag antibody. (C) Interaction between Flag-tagged WT or mutant HSP90α and endogenous GR one hr after IR was visualized by immunoprecipitating with anti-GR antibody and immunoblotting with anti-Flag antibody. (D) Interaction between Flag-tagged HSP90β and GR one hr after IR was visualized by immunoprecipitating with anti-GR antibody and immunoblotting with anti-Flag antibody. (E) C2C12 myotubes transfected with siRNA for either Ctrl or DNA-PKcs were treated with IR (4Gy) and the expression level of p-HSP90α and DNA-PK were visualized by immunoblotting. (F) C2C12 myotubes transfected with a vector encoding WT HSP90α and siRNA for either Ctrl or DNA-PKcs were treated with IR and harvested one hour later. Interaction between GR and HSP90α is shown. (G and H) The effect of IR on the interaction between HSP90α mutants and either endogenous eNOS (G) or AR (H) in C2C12 myotubes is shown.
