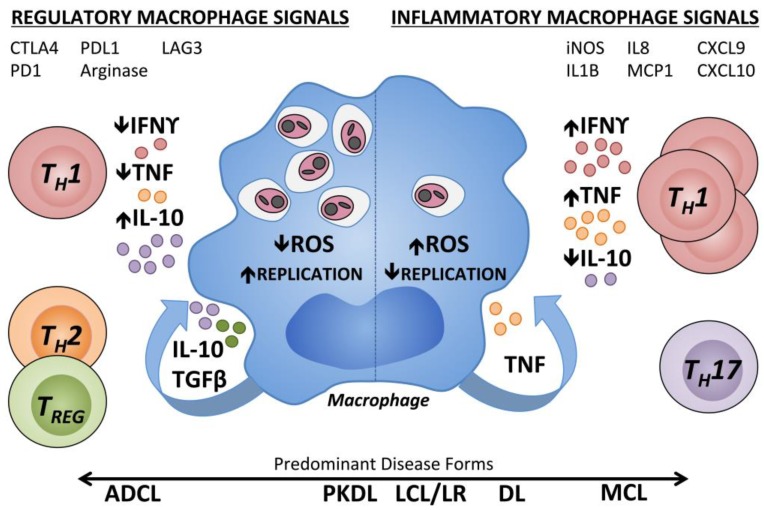
Figure 1.
Immune regulation of human macrophages contributing to different pathologic states during Leishmania infection. Human macrophages parasitized by Leishmania spp. are subject to regulation by cytokines present within the skin at the infection site. Inflammatory Type 1 cytokines, IFNγ and TNF, can synergistically induce ROS production by macrophages, thereby inhibiting Leishmania replication within the phagolysosome. TH17 cells are increased in inflammatory MCL lesions. Chemoattractant transcripts (IL8, MCP1, CXCL9, and CXCL10) and transcripts associated with classical (type 1) macrophage activation (iNOS, IL1β) have been measured in inflammatory CL lesions. In a regulatory environment (left), there are low levels of type 1 cytokines with lack of microbicidal effectors. T cell or macrophage-derived cytokines including type 2 cytokines, IL-10 and TGFβ antagonize the effects of IFNγ and TNF, and enhanced polyamines can result in parasite proliferation. TREG cells can suppress the effects of IFNγ. Inhibitory receptors on CD4+ or CD8+ T cells (CTLA4, PD1, and LAG3) and their counter-ligands (CD80, CD86, and PDL1) are associated with T cell exhaustion. Arginase activity is associated with M2-type non-classical macrophage activation. IFNγ: Interferon γ; TNF: Tumor necrosis factor; IL10: Interleukin 10; TGFβ: Transforming growth factor β; ROS: Reactive oxygen species; ADCL: Anergic diffuse cutaneous leishmaniasis; PKDL: Post Kala-Azar dermal leishmaniasis; LCL: Localized cutaneous leishmaniasis; LR: Leishmania recidivans; DL: Disseminated leishmaniasis; MCL: Mucocutaneous leishmaniasis; DTH: Delayed type 1 hypersensitivity; TREG: T regulatory cell; TH: T helper cell; MCP: Monocyte chemoattractant protein; CXCL: Chemokine (C-X-C motif) ligand; CTLA4: Cytotoxic T-lymphocyte associated protein 4; PD1: Programmed cell death protein 1; LAG3: Lymphocyte activation gene 3; PDL1: Programmed death ligand 1.