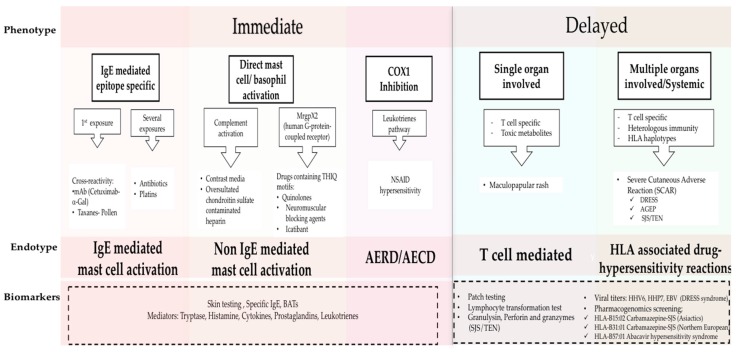
Figure 1.
Phenotypes and Endotypes in drug allergy. The new classification of DHRs is based on phenotypes, endotypes and biomarkers. Phenotypes include immediate and delayed reactions; the clinical presentations of each phenotype are mediated by different immunological mechanisms which are defined by endotypes. Biomarkers are used to identify the Endotypes (dash line box). Adapted from Muraro, Antonella, et al. “Precision Medicine in Allergic Disease–Food Allergy, Drug Allergy, and Anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology.” Allergy (2017) [1]. BAT, basophil activation test; mAb, monoclonal antibody; α-Gal, galactose-alpha-1,3-galactose; NSAID, nonsteroidal anti-inflammatory; AERD, Aspirin Exacerbated Respiratory Disease; AECD, Aspirin Exacerbated Cutaneous Disease; HHV 6, human herpesvirus 6; HHV 7, human herpesvirus 7; EBV, Epstein Barr Virus; DRESS, Drug reaction with Eosinophilia and Systemic Symptoms; AGEP, Acute Generalized Exanthematous Pustulesis; SJS-TEN, Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis.