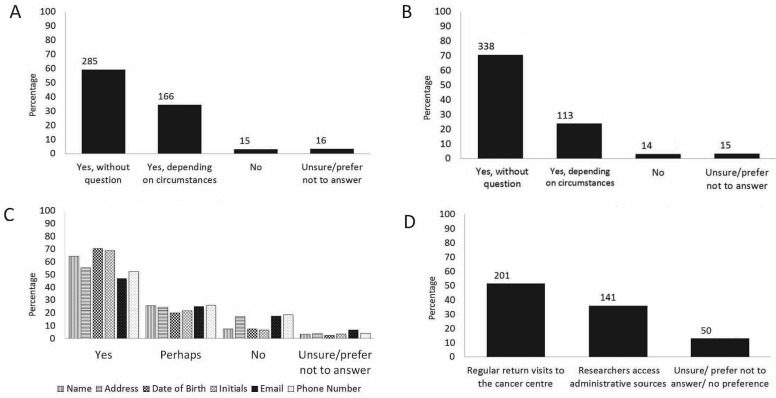
FIGURE 1.
Responses of eligible participants in cohort 1 to selected survey questions. (A) Would you be willing to allow the research team running the clinical trials confidential access to your health information contained in administrative databases? [n]. (B) Would you be willing to allow the research team running the clinical trials to use your initials and full date of birth to match the information from administrative databases? [n]. (C) What information would you allow to be stored securely at the central research coordinating centre? (D) After your treatment was over and it was no longer necessary for you to attend the cancer clinic, how would you prefer to have your long-term follow-up information collected? [n].