Abstract
Background
Hepatocellular carcinoma (HCC) occurs rarely in children and adolescents (C&A), and its clinical characteristics, prognostic factors, and treatment were rarely explored.
Methods
This retrospective study focused on 65 HCC patients aged ≤20 years from August 1994 to August 2012. Cox regression models and Kaplan–Meier curves were used to investigate prognostic factors and compare overall survival (OS), respectively.
Results
We found 61.5% of patients to have multiple tumors, 30.8% to have portal vein tumor thrombus, and 16.9% to have distant metastasis. Diameter of tumors was 10.2 ± 4.1 cm. OS at 5 years was 15.8%. Multivariate analyses showed initial treatment (P < 0.001) to be a predictor for OS. For moderate-stage HCC, the median OS of patients who underwent resection was longer than that of patients who underwent transarterial chemoembolization (TACE) or supportive treatment (ST) (P < 0.001). For advanced-stage HCC, the median OS of patients who underwent TACE was longer than that of patients who underwent ST (P = 0.045).
Conclusions
HCC in C&A tends to be more advanced than that in adults, and resection remains the mainstay of treatment for those patients. Moreover, compared with ST, TACE may benefit C&A with moderate- and advanced-stage HCC, which needs further study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11605-017-3420-3) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Children and adolescents, Prognosis, Resection, Transarterial chemoembolization
Introduction
Liver cancer is the second most common cause of cancer-related deaths worldwide, and there are approximately 850,000 new cases per year worldwide.1,2 Hepatocellular carcinoma (HCC) is the most common type of hepatic malignancy, but only 0.5–1% of those aged ≤20 years suffer from HCC.3
Infection by the hepatitis B virus (HBV) is endemic in China. The incidence of HCC in children and adolescents (C&A) is higher than that for hepatoblastoma, a pattern that is different from that reported in Western countries.4,5 Unlike HCC in adults, which commonly arises in the setting of prolonged chronic hepatitis and cirrhosis,6 tumors in C&A have been observed with HBV infection acquired perinatally or due to inherited metabolic disorders.7,8 Moreover, at the time of diagnosis, HCC in C&A is commonly associated with (i) huge hepatomas, (ii) tumor thrombus in the portal vein, and (iii) distant metastasis.7,9 Taken together, such reports suggest that HCC in C&A may be different to that observed in adults.4,8,10 Hence, discovering the prognostic factors of HCC in C&A may help to improve the diagnosis and treatment of HCC.
Even though HCC in C&A exhibits a more malignant tendency, treatment guidelines are lacking. Resection is considered to be the mainstay of curative therapy for long-term survival of C&A with HCC.3,11–13 Nevertheless, the efficiency of transarterial chemoembolization (TACE), which is the established treatment for unresectable HCC in adults,14–16 for C&A with HCC is controversial.3 More specifically, although promising, experience with TACE in C&A with HCC is limited, with only sporadic reports in the literature.3,10 Moreover, most of those studies have suggested that TACE may have minimal effects on survival,4,17 and only one study showed positive effects in inducing resection of HCC in C&A.18 As a result, the role of TACE for HCC in C&A is still unclear.
In the present study, we analyzed (retrospectively) the clinical and pathologic characteristics of HCC in C&A and evaluated prognostic factors that may help predict survival. Moreover, we compared the outcome of HCC in C&A who underwent different types of treatment to provide evidence for future therapy.
Patients and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (Guangzhou, China).
From 15 August 1994 to 15 August 2012, 72 patients aged ≤20 years were diagnosed with HCC by histological examination or at least two coincidental imaging techniques associated with increased alpha-fetoprotein (AFP) level in our Department of Hepatobiliary Oncology. Patients lost to follow-up <1 month after diagnosis (n = 6) or those who did not have sufficient clinical data (n = 1) were excluded, thereby leaving 65 patients to form the study cohort.
Follow-Up
The follow-up program for patients was every 2–3 months for the first postoperative year and 3–6 months thereafter. “Overall survival” (OS) was defined as the interval (in months) from the date of diagnosis to the date of death. Follow-up ended on 1 August 2016.
Collection of Clinical Data
All clinicopathologic data (age, sex, tumor number, tumor size, distant metastasis, portal vein tumor thrombus, TNM stage) were retrieved from the medical records of Sun Yat-sen University Cancer Center. Laboratory data (HBV infection; levels of AFP, alanine aminotransferase [ALT], aspartate aminotransferase [AST], albumin [ALB], total bilirubin [TBIL]) before surgery were obtained. Tumor stage was determined according to the 7th TNM staging system established by the Union for International Cancer Control and the American Joint Committee on Cancer.19
Statistical Analyses
For continuous variables, data were expressed as mean ± standard deviation and were compared using Student’s t test (two-sided). The Cox proportional hazards model was used for univariate and multivariate analyses. Clinical endpoints were calculated using the Kaplan–Meier method with the log-rank test. Analyses were carried out using the SPSS software program (version 20.0; IBM Corporation, Armonk, NY, USA). P <0.05 (two-tailed test) was considered significant.
Results
Survival and Characteristics of Patients
Median follow-up was 9.1 (range, 1.2–110.2) months. Survival at 1, 3, and 5 years was 50.1, 27.5, and 15.8%, respectively.
Sixty-five patients (52 males, 13 females) with a median age of 16.8 (range, 8–20) years were evaluated (Table 1). Also, 81.5% (53/65) of patients had HBV infection, 61.5% patients had multiple tumors, 16.9% had distant metastasis, and 30.8% had portal vein tumor thrombus. Diameter of tumors was 10.2 ± 4.1 cm, and 90.8% of patients were AFP-positive.
Table 1.
Baseline characteristics of C&A patients with hepatocellular carcinoma (HCC)
| Variable | |
|---|---|
| Age | 17 (8–20) |
| Sex | |
| Female | 13 (20%) |
| Male | 52 (80%) |
| HBV infection | |
| No | 12 (18.5%) |
| Yes | 53 (81.5%) |
| ALT (U/L) | 64.5 ± 81.6 |
| AST (U/L) | 94.3 ± 130.6 |
| ALB (g/L) | 42.9 ± 5.2 |
| TBIL (μmol/L) | 22.4 ± 25.4 |
| Tumor diameter (cm) | 10.2 ± 4.1 |
| AFP (ng/mL) | |
| ≤25 | 6 (9.2%) |
| >25 | 59 (90.8%) |
| Tumor number | |
| Solitary | 25 (38.5%) |
| Multiple | 40 (61.5%) |
| Distant metastasis | |
| No | 54 (83.1%) |
| Yes | 11 (16.9%) |
| Portal vein tumor thrombus | |
| No | 45 (69.2%) |
| Yes | 20 (30.8%) |
| TNM stage | |
| I | 9 (13.8%) |
| II | 8 (12.3%) |
| IIIA | 22 (33.8%) |
| IIIB | 14 (21.5%) |
| IVA | 1 (1.5%) |
| IVB | 11 (16.9%) |
| Initial treatment | |
| ST | 16 (24.6%) |
| TACE | 23 (35.4%) |
| Resection | 26 (40%) |
HBV hepatitis B virus, ALT alanine aminotransferase, AST aspartate transaminase, ALB albumin, TBIL total bilirubin, AFP alpha-fetoprotein, TNM tumor-node-metastasis, ST supportive treatment, TACE transcatheter arterial chemoembolization
TNM Categorization
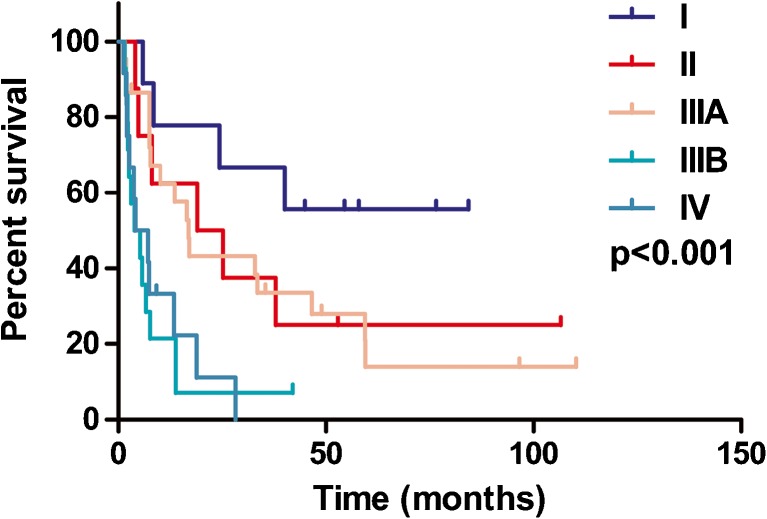
According to the survival curves, we categorized TNM stage into three subgroups (Fig. 1): stage I was “early,” stages II/IIIA were “moderate,” and stages IIIB/IV were “advanced.”
Fig. 1.
Kaplan–Meier curves for OS of HCC patients with different TNM stages
Univariate and Multivariate Analyses for OS in HCC Patients
Univariate and multivariate analyses were undertaken to explore the prognostic factors of HCC patients (Table 2, Fig. 2). Results of the Cox regression hazards model for predictors of OS are shown in Table 2.
Table 2.
Univariate and multivariate analyses of overall survival (OS)
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | ||||
| Female | 1 (reference) | 0.274 | ||
| Male | 1.529 (0.714–3.274) | |||
| HBV infection | ||||
| No | 1 (reference) | 0.306 | ||
| Yes | 1.461 (0.707–3.021) | |||
| Tumor number | ||||
| Solitary | 1 (reference) | 0.882 | ||
| Multiple | 1.068 (0.604–1.886) | |||
| TNM stage | ||||
| Early | 1 (reference) | <0.001 | 0.072 | |
| Moderate | 2.420 (0.835–7.014) | |||
| Advanced | 7.479 (2.503–22.342) | |||
| Initial treatment | ||||
| ST | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| TACE | 0.298 (0.150–0.592) | 0.298 (0.150–0.592) | ||
| Resection | 0.105 (0.048–0.226) | 0.105 (0.048–0.226) | ||
| Distant metastasis | ||||
| No | 1 (reference) | 0.018 | 0.448 | |
| Yes | 2.360 (1.156–4.815) | |||
| Portal vein tumor thrombus | ||||
| No | 1 (reference) | 0.001 | 0.184 | |
| Yes | 2.725 (1.484–5.004) | |||
| Tumor diameter (cm) | ||||
| ≤5 | 1 (reference) | 0.215 | ||
| >5 | 1.912 (0.687–5.323) | |||
| TBIL (μmol/L) | ||||
| ≤20.5 | 1 (reference) | 0.023 | 0.944 | |
| >20.5 | 1.947 (1.094–3.465) | |||
| AST (U/L) | ||||
| ≤45 | 1 (reference) | 0.006 | 0.786 | |
| >45 | 2.365 (1.287–4.346) | |||
| ALT (U/L) | ||||
| ≤40 | 1 (reference) | 0.059 | ||
| >40 | 1.702 (0.979–2.959) | |||
| ALB (g/L) | ||||
| ≤35 | 1 (reference) | 0.457 | ||
| >35 | 0.676 (0.242–1.893) | |||
| AFP (g/L) | ||||
| ≤25 | 1 (reference) | 0.606 | ||
| >25 | 1.309 (0.47–3.649) | |||
HBV hepatitis B virus, TNM tumor-node-metastasis, ST supportive treatment, TACE transcatheter arterial chemoembolization, TBIL total bilirubin, AST aspartate transaminase, ALT alanine aminotransferase, ALB albumin, AFP alpha-fetoprotein
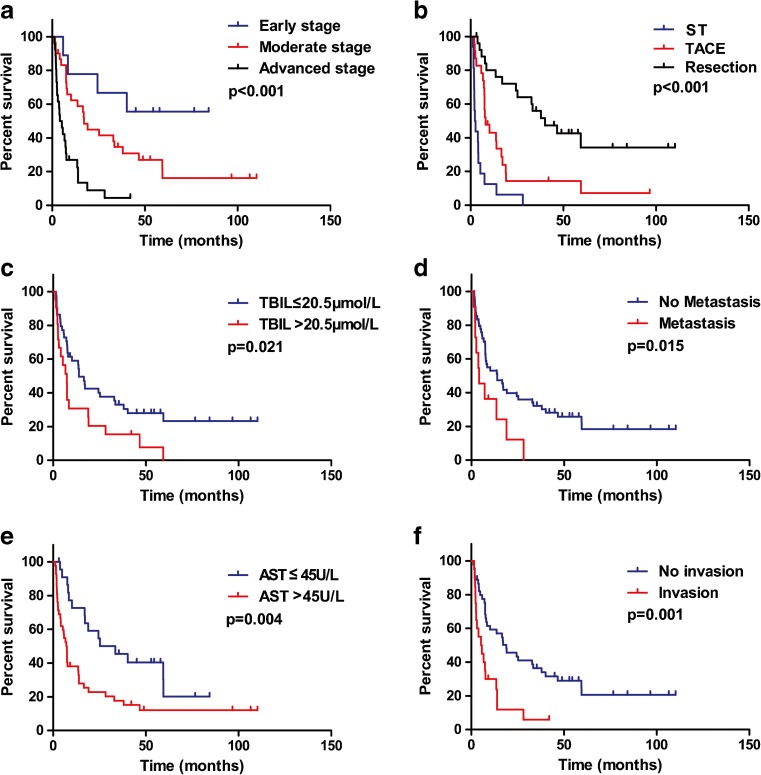
Fig. 2.
Univariate analyses of prognostic factors using Kaplan–Meier survival curves. a Early, moderate, or advanced stage. b ST, TACE, or resection. c TBIL ≤20.5 or >20.5 μmol/L. d Metastasis: no or yes. e AST ≤45 or >45 U/L. f Portal vein tumor thrombus: no or yes
Univariate analyses showed that TNM stage (moderate stage vs. early stage: HR 2.420, 95% confidence interval [CI] 0.835–7.014; advanced stage vs. early stage: HR 7.479, 95% CI 2.503–22.342, P < 0.001), initial treatment (TACE vs. supportive treatment [ST]: HR 0.298, 95% CI 0.150–0.592; resection vs. ST: HR 0.105, 95% CI 0.048–0.226, P < 0.001), metastasis (HR 2.360, 95% CI 1.156–4.815, P = 0.015), portal vein tumor thrombus (HR 2.725, 95% CI 1.484–5.004, P = 0.001), TBIL level (HR 1.947, 95% CI 1.094–3.465, P = 0.021), and AST level (HR 2.365, 95% CI 1.287–4.346, P = 0.004) were associated with OS. Multivariate analyses showed only initial treatment allocation (TACE vs. ST: HR 0.298, 95% CI 0.150–0.592; resection vs. ST: HR 0.105, 95% CI 0.048–0.226, P = 0.001) to be a predictor of OS.
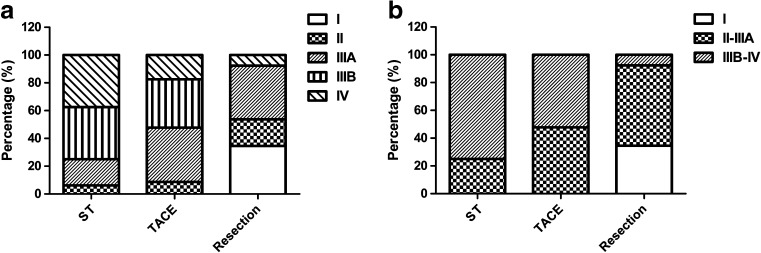
To further investigate the relationship between the option of initial treatment and OS, we found that more patients with early or moderate stage received surgery, while a high proportion of the patients with moderate or advanced stage received TACE or ST (P < 0.001, Fig. 3). These indicated that the uneven distribution of TNM stage may influence the OS of patients undergoing different types of initial treatment.
Fig. 3.
a Distribution of TNM stage for HCC in C&A was different for different treatment types (χ 2 < 0.001). b Distribution of combined TNM stage for HCC in C&A was different for different treatments (χ 2 < 0.001)
Survival
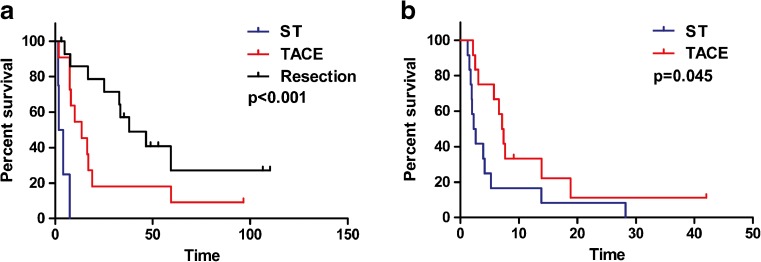
To exclude the influence of the different distribution of TNM stage on initial treatment subgroups, we compared the OS of patients at moderate or advanced stages that underwent different types of treatment. Kaplan–Meier analyses showed that, at the moderate stage, the median OS of patients who underwent resection was longer than that of patients who underwent other types of treatment (resection vs. TACE vs. ST: 38.0 vs. 13.6 vs. 1.8 months, P < 0.001, Fig. 4a). At the advanced stage, the OS of patients who underwent TACE was longer than that of patients who underwent ST (TACE vs. ST: 7.1 vs. 2.3 months, P = 0.045, Fig. 4b).
Fig. 4.
a Kaplan–Meier curves for OS of HCC patients at the moderate stage. Median OS of patients who underwent resection was longer than that of patients who underwent TACE or ST (38.0, 13.6, and 1.8 months, respectively, P < 0.001). b Kaplan–Meier curves for OS of HCC patients with advanced HCC. Median OS of patients who underwent TACE was longer than that of patients who underwent ST (7.1 vs. 2.3 months, P = 0.045). The black curve, red curve, and blue curve represent patients who underwent resection, TACE, and ST, respectively
Discussion
The present study demonstrated that C&A with HCC tend to have advanced disease, and initial treatment allocation was found to be an independent prognostic factor. Resection could achieve long-term survival at the moderate stage compared with other types of treatment. Moreover, compared with ST, TACE could have beneficial effects on OS for the advanced stage of HCC.
Several differences with respect to clinical characteristics and outcomes between HCC in C&A and that in adults have been reported.4,20 Our results identified HBV infection to be present in 81.5% of C&A with HCC, which is similar to the prevalence of adult HCC in our hospital.21,22 In accordance with other studies, we found that HCC was more common in males than in females (4:1).3,5 AFP is a useful diagnostic marker for HCC, and roughly 50–70% of adults with HCC have increased levels of AFP,3,23 compared with >90% in C&A in our study. Moreover, some studies have suggested higher serum levels of AFP to be correlated with worse outcome in HCC.24–26 We showed that 81.5% of C&A with HCC had AFP >400 ng/mL, which suggests that C&A tend to present with more advanced disease compared with adults. However, we did not find a significant difference in OS between the two groups (AFP >400 vs. ≤400 ng/mL; median OS 7.7 vs. 28.2 months, P = 0.075), which might be due to the small sample size. Recent studies have shown serum levels of AFP to be associated with tumor diameter,27–29 as did our data (r = 0.261, P = 0.035) (Fig. S1), with 58 (89.2%) patients having a tumor diameter >5 cm and 34 (52.3%) patients having a tumor diameter ≥10 cm. Moreover, multiple nodules were identified in 40 (61.5%) C&A patients. Recent studies have shown the prevalence of distant metastasis and tumor thrombus in the portal vein to be higher in C&A than that in adults, which was correlated with worse outcomes.1,3 , 4 We also showed that 11 (16.9%) patients had distant metastasis and 20 (30.8%) patients had tumor thrombus in the portal vein upon first hospital admission. Moreover, our findings showed that HCC in C&A carried a dismal 5-year OS of 15.8%, which is lower than that observed in adults.8,30 These findings suggest that HCC in C&A tends to present with more advanced disease than that in adults.
Even though HCC in C&A tends to be more malignant, treatment guidelines on HCC management are lacking.3,13 Using univariate and multivariate analyses, we identified initial treatment allocation to be an independent prognostic factor. Different types of initial treatment affected the prognosis of HCC in C&A markedly, with the median survival for resection, TACE, and ST being 40.1, 8.0, and 2.3 months, respectively (P < 0.001, Fig. 2b). If stratified by TNM stage, moderate-stage patients who underwent resection had better outcome than those who underwent TACE or those who did have ST (P < 0.001, Fig. 4a). For patients with advanced disease, those who underwent TACE had better outcome than those who did have ST (P < 0.05, Fig. 4b). TACE has been shown to improve survival for unresectable HCC in adults,31,32 but its role in C&A with HCC is not known.3,17 Thus, our study showed that compared with ST, TACE might have an effect on unresectable HCC in C&A. However, it should be noted that the difference in OS between ST and TACE might be based on differences in the clinical condition of patients due to the retrospective nature of the study, and prospective studies are needed to confirm those findings.
In the current retrospective study, none of the patients underwent liver transplantation. Some recent studies showed that liver transplantation might be an effective treatment option although the literature is sparse. The studies of Pham et al. and Malek et al. suggested that liver transplantation for HCC in C&A could result in excellent long-term survival, even though the lesions were well outside the Milan and UCSF criteria.33,34 However, more studies are needed to verify transplantation as a therapeutic intervention for HCC in C&A. Chemotherapy has little effect in improving survival.3,35 Preliminary results from the Pediatric Oncology Group (POG) study showed that a cisplatin-based chemotherapy regimen might benefit children with resectable HCC.20 However, two subsequent prospective studies from the International Childhood Liver Tumor Study Group (SIOPEL 2 and SIOPEL 3) found that intensification of platinum agents did not result in improved survival.36 Moreover, the data about ablative therapies and sorafenib in C&A with HCC is lacking, which needs further study. At present, there is no standard clinical guideline for HCC in C&A, and more prospective studies should be conducted in the future.
In addition, two patients were found to have extrahepatic metastasis after laparotomy (one with a nodule in the gastric omentum and the other with a nodule in the diaphragm). When the tumors were removed successfully, the two patients died at 3.7 and 13.5 months, respectively.
Our study had three main limitations. First, this was a retrospective review of data collected from a single cancer center with a small sample size. Hence, we provided low-grade evidence for future therapy.37 Second, apart from one patient, the age of the other patients was 10–20 years old. Therefore, the efficacy of TACE in HCC patients under the age of 10 years must be evaluated further. Third, the efficacy of initial TACE therapy for patients with unresectable HCC might be skewed by subsequent treatments. Nevertheless, this can be deciphered by the better treatment response for initial TACE therapy because the subsequent treatments depended mostly on tumor burden and liver function.31
Conclusions
Compared with HCC in adults, HCC in C&A tends to be more advanced, which results in worse survival in C&A. Initial treatment allocation was found to be an independent prognostic factor for HCC in C&A. Consistent with other studies, our study showed that resection is the recommended choice for early- and moderate-stage HCC when technically feasible. Moreover, we found that, compared with ST, TACE may benefit C&A with moderate- and advanced-stage HCC.
Electronic Supplementary Material
(DOCX 20 kb)
(GIF 8 kb)
Acknowledgements
We would like to thank ELIXIGEN company for English language editing.
Author contributions
Juncheng Wang, Yize Mao, Xiangming Lao, and Shengping Li were responsible for the study’s conceptualization; Juncheng Wang and Yize Mao for the formal analysis; Juncheng Wang, Yize Mao, Yongcheng Liu, Zhenxin Chen, Minshan Chen, Xiangming Lao, and Shengping Li for the investigation; Xiangming Lao and Shengping Li for data curation; Juncheng Wang and Yize Mao for the writing of the original draft; all authors for the writing, review, and editing; Juncheng Wang, Yize Mao, Yongcheng Liu, Zhenxin Chen, and Minshan Chen for the visualization; Xiangming Lao and Shengping Li for the supervision; and Xiangming Lao and Shengping Li for the project administration.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding Sources
No specific funding was disclosed.
Footnotes
Juncheng Wang and Yize Mao contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s11605-017-3420-3) contains supplementary material, which is available to authorized users.
Contributor Information
Xiangming Lao, Email: laoxm@sysucc.org.cn.
Shengping Li, Email: lishp@sysucc.org.cn.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nature Reviews Disease Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Kelly D, Sharif K, Brown RM, Morland B. Hepatocellular carcinoma in children. Clinics in Liver Disease. 2015;19(2):433–47. doi: 10.1016/j.cld.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XF, Liu XM, Wei T, Liu C, Li MX, Long ZD, et al. Clinical characteristics and outcome of hepatocellular carcinoma in children and adolescents. Pediatric Surgery International. 2013;29(8):763–70. doi: 10.1007/s00383-013-3334-4. [DOI] [PubMed] [Google Scholar]
- 5.Hung GY, Horng JL, Yen HJ, Lee CY, Lin LY. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. Journal of Hepatology. 2015;63(6):1390–6. doi: 10.1016/j.jhep.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. The New England Journal of Medicine. 2011;365(12):1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.McAteer JP, Goldin AB, Healey PJ, Gow KW. Hepatocellular carcinoma in children: epidemiology and the impact of regional lymphadenectomy on surgical outcomes. Journal of Pediatric Surgery. 2013;48(11):2194–201. doi: 10.1016/j.jpedsurg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology (Baltimore, Md) 2003;38(3):560–6. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- 9.von Schweinitz D. Management of liver tumors in childhood. Seminars in Pediatric Surgery. 2006;15(1):17–24. doi: 10.1053/j.sempedsurg.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Hadzic N, Finegold MJ. Liver neoplasia in children. Clinics in Liver Disease. 2011;15(2):443–62. doi: 10.1016/j.cld.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 11.McAteer JP, Goldin AB, Healey PJ, Gow KW. Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatric Transplantation. 2013;17(8):744–50. doi: 10.1111/petr.12144. [DOI] [PubMed] [Google Scholar]
- 12.Hiyama E. Current therapeutic strategies for childhood hepatic malignant tumors. International Journal of Clinical Oncology. 2013;18(6):943–5. doi: 10.1007/s10147-013-0607-9. [DOI] [PubMed] [Google Scholar]
- 13.Allan BJ, Wang B, Davis JS, Parikh PP, Perez EA, Neville HL, et al. A review of 218 pediatric cases of hepatocellular carcinoma. Journal of Pediatric Surgery. 2014;49(1):166–71. doi: 10.1016/j.jpedsurg.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Llovet JM, Castells A, Montana X, Bru C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology (Baltimore, Md) 1998;27(6):1578–83. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JH. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology (Baltimore, Md) 2016 doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2002;20(22):4459–65. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Chen JC, Chen CC, Chen WJ, Lai HS, Hung WT, Lee PH. Hepatocellular carcinoma in children: clinical review and comparison with adult cases. Journal of pediatric surgery. 1998;33(9):1350–4. doi: 10.1016/S0022-3468(98)90005-7. [DOI] [PubMed] [Google Scholar]
- 18.Malogolowkin MH, Stanley P, Steele DA, Ortega JA. Feasibility and toxicity of chemoembolization for children with liver tumors. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2000;18(6):1279–84. doi: 10.1200/JCO.2000.18.6.1279. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu-Mares W, Douglass EC, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2002;20(12):2789–97. doi: 10.1200/JCO.2002.06.155. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Journal of hepatology. 2015;63(1):122–30. doi: 10.1016/j.jhep.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2013;31(4):426–32. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 23.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7(4):350–91. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World Journal of Surgical Oncology. 2013;11:212. doi: 10.1186/1477-7819-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis—hepatocellular carcinoma and other disorders. Clinics in Liver Disease. 2015;19(2):309–23. doi: 10.1016/j.cld.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–94. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 27.Abbasi A, Bhutto AR, Butt N, Munir SM. Corelation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. JPMA The Journal of the Pakistan Medical Association. 2012;62(1):33–6. [PubMed] [Google Scholar]
- 28.Toro A, Ardiri A, Mannino M, Arcerito MC, Mannino G, Palermo F, et al. Effect of pre- and post-treatment alpha-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: a retrospective study. BMC Surgery. 2014;14:40. doi: 10.1186/1471-2482-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, et al. Value of alpha-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World Journal of Gastroenterology : WJG. 2013;19(11):1811–9. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Annals of Surgery. 1998;228(4):479–90. doi: 10.1097/00000658-199810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi M, Lu LG, Fang WQ, Guo RP, Chen MS, Li Y, et al. Roles played by chemolipiodolization and embolization in chemoembolization for hepatocellular carcinoma: single-blind, randomized trial. Journal of the National Cancer Institute. 2013;105(1):59–68. doi: 10.1093/jnci/djs464. [DOI] [PubMed] [Google Scholar]
- 32.Zhou ZG, Zheng XR, Zhou Q, Shi M, Zhang YJ, Guo RP, et al. Impact of oral anti-hepatitis B therapy on the survival of patients with hepatocellular carcinoma initially treated with chemoembolization. Chin J Cancer. 2015;34(1):14. doi: 10.1186/s40880-015-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham TA, Gallo AM, Concepcion W, Esquivel CO, Bonham CA. Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surgery. 2015;150(12):1150–8. doi: 10.1001/jamasurg.2015.1847. [DOI] [PubMed] [Google Scholar]
- 34.Malek MM, Shah SR, Atri P, Paredes JL, DiCicco LA, Sindhi R, et al. Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery. 2010;148(4):778–82. doi: 10.1016/j.surg.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2002;20(12):2798–804. doi: 10.1200/JCO.2002.06.102. [DOI] [PubMed] [Google Scholar]
- 36.Murawski M, Weeda VB, Maibach R, Morland B, Roebuck DJ, Zimmerman A, et al. Hepatocellular carcinoma in children: does modified platinum- and doxorubicin-based chemotherapy increase tumor resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology. 2016;34(10):1050–6. doi: 10.1200/JCO.2014.60.2250. [DOI] [PubMed] [Google Scholar]
- 37.Koh C, Zhao X, Samala N, Sakiani S, Liang TJ, Talwalkar JA. AASLD clinical practice guidelines: a critical review of scientific evidence and evolving recommendations. Hepatology (Baltimore, Md) 2013;58(6):2142–52. doi: 10.1002/hep.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)
(GIF 8 kb)