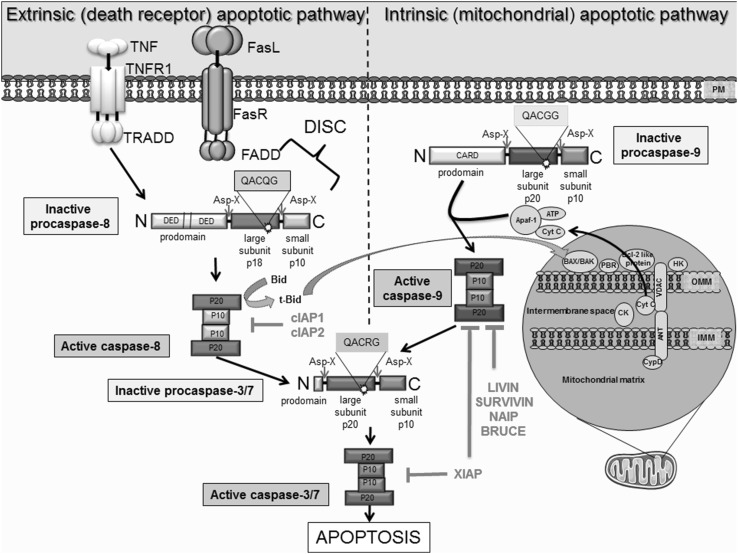
Fig. 1.
Schematic illustration of the extrinsic and intrinsic apoptotic pathways, as well as the inhibitory effect of various IAPs on pro-apoptotic molecules. Extrinsic apoptotic pathway initiated by binding of death ligands, such as FasL or tumor necrosis factor (TNF) to death receptors located on the plasma membrane. This reaction is followed by the recruitment and binding of molecules like Fas-associated death domain protein (FADD) or tumor necrosis factor receptor type 1-associated death domain protein (TRADD) to the cytosolic domain of death receptors. Death-inducing signaling complex (DISC) is formed by death receptor, FADD and caspase 8. DISC formation initiates the signal transduction that culminates in apoptosis via caspase 3/7 activation. Active caspases can enhance apoptosis via cleavage of Bid to tBid; a cross-talk facilitator that mediates the mitochondrial amplification loop. The truncated Bid (t-Bid) promotes the release of cytochrome c, via Bax, in mitochondria. The intrinsic pathway, is initiated within at the outer mitochondrial membrane (OMM) in response to cellular stress. As a result, these mediate mitochondrial permeability via interaction ‘pro-apoptotic’ Bcl-2 proteins to stimulate release of cytochrome c and SMAC, which bind and inhibit IAPs. Cytochrome c, Apaf-1 and ATP binds to pro caspase 9 leading to apoptosome formation and activation of caspase 9, which in turn activate caspase 3 permitting the cell to proceed to apoptosis. IAPs are endogenous inhibitors of apoptosis identified in humans. The family members XIAP, cIAP1, cIAP2, NAIP, Livin and Survivin and BRUCE can bind caspases to block apoptosis. Importantly, their dysregulated expression is associated with cancer and chemoresistance