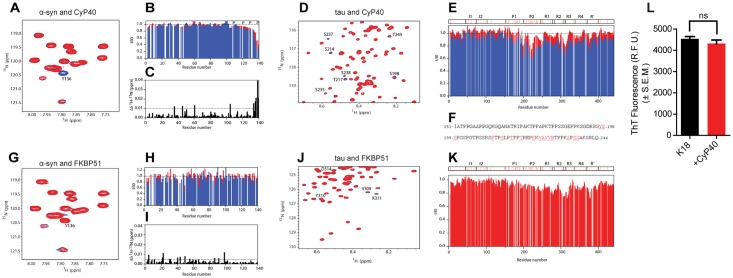
Fig 6. Cyclophilin 40 (CyP40) binds proline-containing regions of tau and α-synuclein (α-syn).
(A) A selected region of the 2D [1H-15N]–heteronuclear single quantum coherence (HSQC) experiment of 15N-labeled α-syn before (blue) and after (red) addition of CyP40 (molar ratio 1:15). Y136 is marked. (B) Normalized residue-specific nuclear magnetic resonance (NMR) intensities in the presence of a 5-fold (red) and 15-fold (blue) excess of Cyp40; the location of proline residues is indicated. (C) Combined [1H-15N] chemical shift perturbation analysis upon addition of CyP40 to α-syn. (D) A selected region of 2D [1H-15N]-HSQC experiment of tau before (blue) and after (red) addition of CyP40 (molar ratio 1:10) is shown. Residues displaying significant intensity loss are labeled. (E) Normalized residue-specific NMR intensities of tau in the presence of a 5-fold (red) and 10-fold (blue) excess of CyP40; tau’s domain organization is shown, and proline residues are marked with red. (F) Amino acid sequence of the proline-rich regions P1 (top) and P2 (bottom); residues significantly broadened upon addition of CyP40 are colored red. (G) A selected region of the 2D [1H-15N]-HSQC experiment of 15N-labeled α-syn before (blue) and after (red) addition of FK-506 binding protein (FKBP) 51 (molar ratio 1:10) is displayed. (H) Normalized residue-specific NMR intensities in the presence of a 5-fold (red) and 10-fold (blue) excess of FKBP51. (I) Combined [1H-15N] chemical shift perturbation analysis upon addition of FKBP51 to α-syn. (J) Selected region of a 2D [1H-15N]-HSQC experiment of tau before (blue) and after (red) addition of FKBP51 (molar ratio 1:10). Final concentrations of CyP40 and FKBP51 for NMR experiments are 200 μM. (K) Tau residue-specific NMR intensities in presence of a 10-fold excess of FKBP51. (L) Thioflavin T fluorescence of K18 tau aggregates in the presence or absence of CyP40 (unpaired t test, p > 0.05, n = 3). The numerical data used in panel 6L can be found in S1 Data.