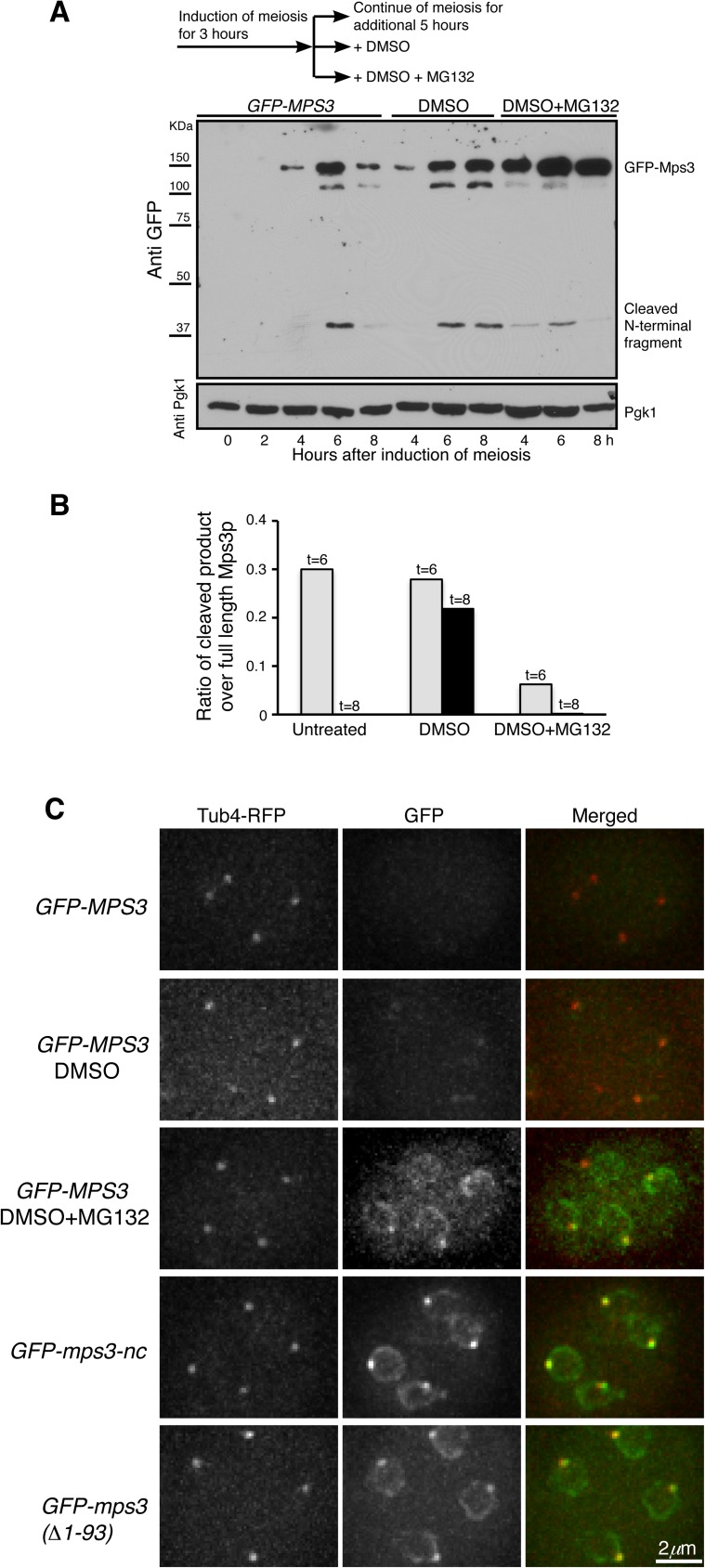
Fig 5. Proteasome-dependent cleavage of Mps3 at its N-terminal domain.
(A) The proteasome activity is required for Mps3 cleavage. Yeast cells were induced to undergo synchronous meiosis for 3 h, and then the culture was split into three fractions as shown in the diagram on the top of this panel. Protein extracts were prepared from cells at indicated times for western blot and probed by an anti-GFP antibody. Note that the addition of MG132, but not DMSO, inhibited Mps3 cleavage. Strain HY4430. (B) Quantification of Mps3 cleavage in cells as shown in A. Gray bars show the values obtained 6 h after induction of meiosis; black bars show 8 h in DMSO and DMSO+MG132 treated samples. (C) Cytological evidence of Mps3 cleavage during yeast meiosis. Yeast cells were induced to undergo meiosis for about 12 h, and fluorescence microscopy was performed to observe GFP-Mps3 and Tub4-RFP localization in cells that had completed meiosis. Projected images from 12 z-stacks were shown. Tub4-RFP marks the SPB. In these strains, GFP was fused to the N-terminus of Mps3. The GFP-MPS3 constructs were under the control of the DMC1 promoter. Note that treatment of cells with MG132 inhibited Mps3 cleavage in wild-type cells, whereas mps3-nc and mps3(Δ1–93) cells lacked cleavage. Strains HY4430, HY4373 and HY4978.