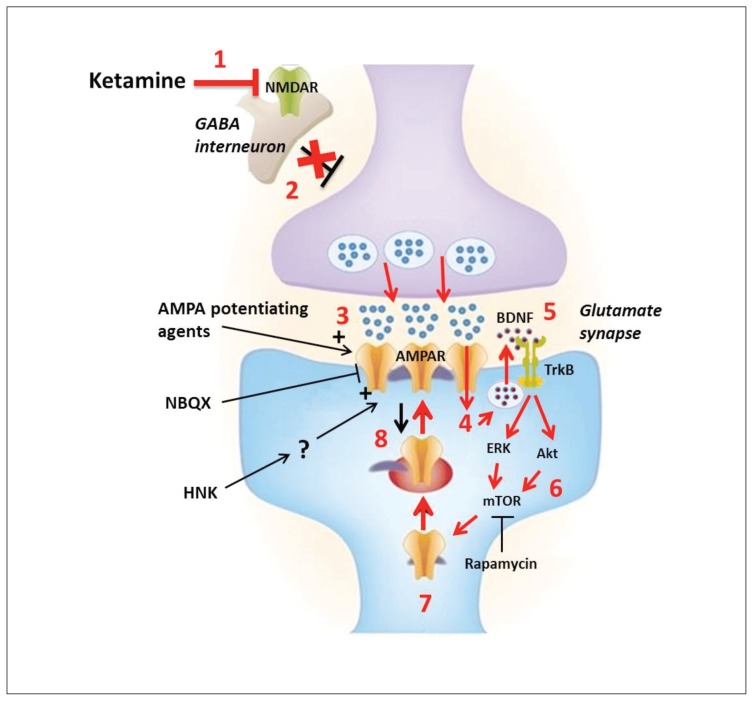
Fig. 1.
Convergent theory of ketamine’s mechanism of action in depression. 1) At low doses ketamine is thought to preferentially bind to and inhibit N-methyl-d-aspartate receptors (NMDARs) on γ-aminobutyric acid (GABA)ergic interneurons. 2) This results in reduced excitability of these inhibitory interneurons, which in turn causes disinhibition of glutamatergic neurons. 3) Increased depolarization of the presynaptic neuron leads to a surge of glutamate release, as reported in the medial prefrontal cortex (mPFC). 3) Released glutamate binds to and activates postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which conduct Na+ and Ca2+ into the cell. 4) The calcium influx and depolarization activates voltage-gated calcium channels (VDCCs). 5) The high local intracellular concentration of Ca2+ triggers the activity-dependent vesicular release of brain-derived neurotrophic factor (BDNF) into the synaptic space. 6) BDNF binds to and activates its surface receptor, tropomyosin receptor kinase B (TrkB), which activates 2 major downstream signalling cascades involving MEK–ERK and PI3K-Akt. These 2 pathways converge onto the mechanistic target of rapamycin (mTOR), which is a key regulator of protein synthesis and synaptic plasticity. 7) These events lead to disinhibition of synaptic protein translation (e.g., GluR1–2, PSD95, synapsin1) as well as BDNF, in part through the supressed phosphorylation of eukaryotic elongation factor 2 (eEF2). 8) These newly synthesized proteins are then inserted into the postsynaptic density, leading to further increases in AMPAR-mediated synaptic transmission and dendritic spine density, thus causing massive synaptogenesis. This series of events is believed to restore normal connectivity between key brain regions, such as the PFC and limbic structures like the hippocampus and amygdala. The AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) completely abolishes ketamine’s antidepressant actions, supporting the role of AMPA receptors in mediating these effects. On the other hand, AMPA potentiating agents could represent a more targeted strategy of obtaining ketamine-like antidepressant effects. The ketamine metabolite, hydroxynorketamine (HNK), has been recently shown to facilitate AMPAR-mediated transmission, but the exact molecular target and mechanism of action of HNK remain unknown. Figure adapted with permission from from Sanacora and Schatzberg.61