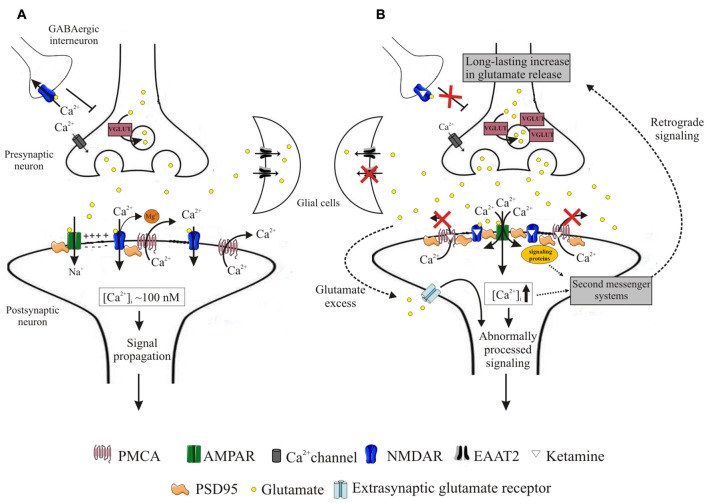
Figure 9.
Schematic representation of events induced by ketamine. During neurotransmission, presynaptically released glutamate binds to both AMPA and NMDA receptors. Activation of AMPA receptors depolarizes postsynaptic membrane, driving Mg2+ out of the NMDA receptor pore. This allows for Ca2+ entry to the cell, from whence it is subsequently rapidly extruded into the synaptic cleft by high-affinity PMCA. This allows for tight control of intrasynaptic [Ca2+]c rises and propagation of unaltered downstream signals (A). Blockage of NMDA receptors by ketamine induces massive Ca2+ influx through AMPA receptors. Ketamine also inhibits PMCA, depleting cellular Ca2+-clearing potency and leading to elevated [Ca2+]c within the synapse. This triggers formation of NMDAR/PSD95/PMCA complexes, putatively located close to the Ca2+ entry sites and acting to restore resting [Ca2+]c. These complexes may further recruit signaling molecules but transmission of aberrant retrograde signal leads to a long-lasting increase in presynaptic glutamate secretion facilitated by overexpression of VGLUTs. Once released, glutamate is not effectively cleared due to downregulation of glial EAAT2 in what seems to constitute another adaptive change to restore defective postsynaptic NMDA receptor-mediated signaling. The excess of glutamate in the synaptic cleft may activate extra-synaptic receptors and together with dysregulated Ca2+ homeostasis contribute to the propagation of abnormal signals (B).