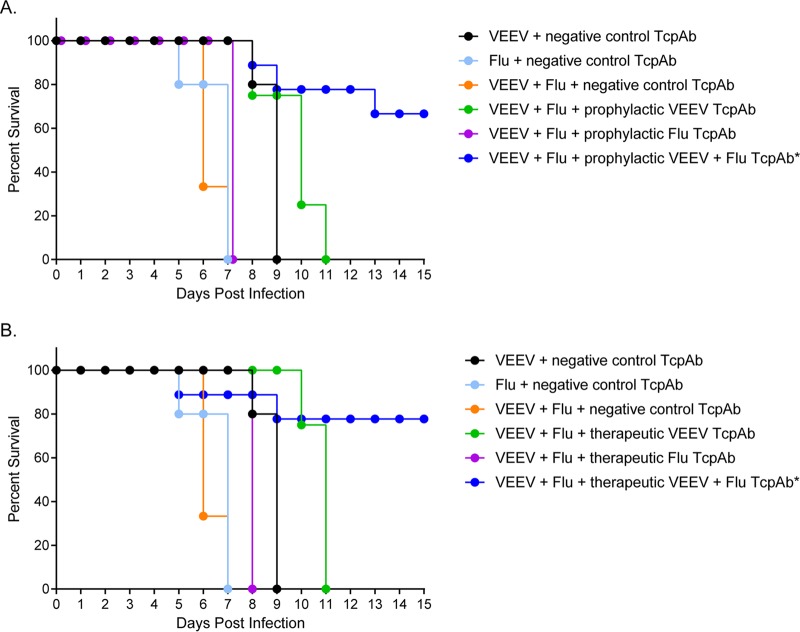
FIG 10.
Efficacy of combined TcPAb treatment against aerosol coinfection with influenza virus and VEEV. Six-week-old DBA2 mice were treated with the negative-control TcPAb or treated either prophylactically or therapeutically with anti-VEEV TcPAb (V3000 nt3A AMT) or anti-influenza virus TcPAb (H1N1-H3N2) before lethal aerosol challenge with VEEV and influenza virus (H1N1). (A) Mice treated prophylactically received two intraperitoneal doses of either 100 μg anti-VEEV, 200 μg anti-influenza virus TcPAb, or a combination of both TcPAbs, with the first dose 12 h before challenge and the second dose at 48 h postchallenge. (B) Mice treated therapeutically received two intraperitoneal doses of 100 μg anti-VEEV, 200 μg anti-influenza virus TcPAb, or a combination of both TcPAbs, with the first dose 12 h after challenge and the second dose 48 h after challenge. Statistical significance was determined by a Mantel-Cox log rank test. *, P < 0.001 versus the negative-control TcPAb. In negative-control groups, the survival time for each virus singly was significantly longer than that for combined infection (P < 0.01) (n = 9 for all groups except for mice that received only the single anti-VEEV or anti-influenza virus TcPAb, where n = 4).