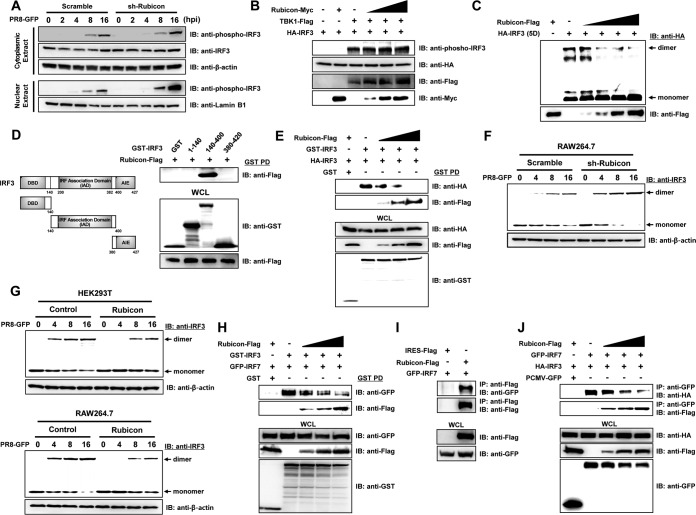
FIG 6.
Rubicon inhibits dimerization of IRF3. (A) Control (Scramble) and Rubicon knockdown (sh-Rubicon) RAW264.7 cells were infected with PR8-GFP (MOI = 2) and harvested at the indicated times (0, 2, 4, 8, and 16 hpi). Cytoplasmic and nuclear extracts were then subjected to immunoblotting with anti-phospho-IRF3 and -IRF3 antibodies. β-Actin and lamin B1 were used to confirm equal loading of proteins. (B) TBK1-Flag and HA-IRF3 were coexpressed, along with increasing levels of Rubicon-Myc, in HEK293T cells. The lysates were then subjected to immunoblotting with anti-phospho-IRF3, -HA, -Flag, and -Myc antibodies. (C) An HA-tagged active phosphomimetic mutant of IRF3 (HA-IRF3-5D) and increasing levels of Rubicon-Flag were overexpressed in HEK293T cells. The cell lysates were then resolved in native polyacrylamide gels, and both monomeric and dimeric forms of IRF3-5D were detected using an anti-HA antibody. Rubicon-Flag was detected by immunoblotting with an anti-Flag antibody. (D) Schematic representation of GST-IRF3 constructs comprising amino acids 1 to 140 (containing the DBD), amino acids 140 to 400 (containing the IAD), and amino acids 380 to 427 (containing the AIE). HEK293T cells were transfected with the indicated GST-IRF3 constructs and Rubicon-Flag plasmids. GST pulldown (PD) was then conducted, followed by immunoblotting with an anti-Flag antibody. WCL were immunoblotted with anti-GST and -Flag antibodies. (E) Control vector (GST), HA-IRF3, or GST-IRF3 was coexpressed with increasing levels of Rubicon-Flag in HEK293T cells. Immunoprecipitation of GST-IRF3 from the cell lysates was conducted in a GST pulldown assay, followed by immunoblotting with anti-HA and -Flag antibodies. WCL were immunoblotted with anti-HA, -Flag, and -GST antibodies. (F and G) Together with control cells (Scramble and Control), Rubicon knockdown RAW264.7 cells (sh-Rubicon) or Rubicon-overexpressing HEK293T and RAW264.7 cells (Rubicon) were infected with PR8-GFP. At the indicated times (0, 4, 8, and 16 hpi), cells were harvested and subjected to native polyacrylamide gel electrophoresis to detect monomeric and dimeric forms of IRF3, followed by immunoblotting with an anti-IRF3 antibody. β-Actin was used to confirm equal loading of proteins. (H) Control vector (GST), GFP-IRF7, and GST-IRF3 were coexpressed with increasing levels of Rubicon-Flag in HEK293T cells. Immunoprecipitation of GST-IRF3 from the cell lysates was conducted in a GST pulldown assay, followed by immunoblotting with anti-GFP and -Flag antibodies. WCL were immunoblotted with anti-GFP, -Flag, and -GST antibodies. (I) Cell lysates of HEK293T cells that were overexpressed with control vector (IRES-Flag), Rubicon-Flag, and GFP-IRF7 were subjected to immunoprecipitation with an anti-Flag antibody, followed by immunoblotting with an anti-GFP antibody. WCL were immunoblotted with anti-Flag and -GFP antibodies. (J) HEK293T cells were cotransfected with control vector (PCMV-GFP), GFP-IRF7, HA-IRF3, and increasing doses of Rubicon-Flag plasmids. IRF7-binding proteins were detected by immunoprecipitation with anti-GFP antibody, followed by immunoblotting with anti-HA and anti-Flag. WCL were immunoblotted with anti-HA, anti-Flag, and anti-GFP antibodies.