ABSTRACT
Methicillin-susceptible Staphylococcus aureus (MSSA) bloodstream infections (BSIs) often lead to severe complications despite the availability of effective antibiotics. It remains unclear whether elevated vancomycin MICs are associated with worse outcomes. We conducted a 2-year retrospective cohort study (n = 252) of patients with MSSA BSIs at a tertiary care hospital. We defined reduced vancomycin susceptibility (RVS) as a Microscan MIC of 2 mg/liter. All strains were genotyped (spa) and assessed for agr functionality. Multivariable logistic regression models were used to examine the impact of RVS phenotype and strain genotype on 30-day all-cause mortality and complicated bacteremia (metastatic spread, endovascular infection, or duration ≥3 days). One-third of patients (84/252) were infected with RVS isolates. RVS Infections were more frequently associated with metastatic or embolic sites of infection (36% versus 17%, P < 0.001), and endovascular infection (26% versus 12%, P = 0.004). These infections occurred more often in patients with fewer underlying comorbidities (Charlson comorbidity index of ≥3 [73% versus 88%, P = 0.002]). Genotyping identified 127 spa-types and 14 Spa-clonal complexes (Spa-CCs). Spa-CC002 and Spa-CC008 were more likely to exhibit the RVS phenotype versus other Spa-CCs (OR = 2.2, P < 0.01). The RVS phenotype was not significantly associated with 30-day mortality; however, it was associated with complicated bacteremia (adjusted odds ratio of 2.35 [range, 1.26 to 4.37]; P = 0.007) in adjusted analyses. The association of RVS strains with complicated infection and fewer underlying comorbidities suggests the phenotype as a potential marker of strain virulence in MSSA BSIs. The RVS phenotype itself was not a significant predictor of mortality in this patient cohort. Further studies are necessary to explore this host-pathogen relationship.
KEYWORDS: MSSA bacteremia, reduced vancomycin susceptibility
INTRODUCTION
Staphylococcus aureus is a dynamic pathogen causing a broad range of clinical syndromes, from localized skin-and-soft tissue infections to invasive disease (1). S. aureus bloodstream infections (SAB) often result in endovascular seeding and are frequently associated with poor outcomes, including 30-day mortality estimated at 20 to 40% (2–6). While bloodstream infections (BSIs) caused by methicillin-resistant S. aureus (MRSA) have historically attracted the most attention, methicillin-sensitive S. aureus (MSSA) is responsible for the majority of S. aureus BSIs (7, 8).
Morbidity and mortality in patients with S. aureus BSI are due to a complex interplay between timely and effective treatment, host-pathogen interactions, underlying patient characteristics, and pathogen features such as antimicrobial drug resistance (9–12). The mechanism for reduced vancomycin susceptibility (RVS) isolates is poorly understood and is distinct from genetic alterations seen in vancomycin resistance, which involves acquisition of the vanA operon (13). Reduced vancomycin susceptibility is thought to be accompanied by thickening of the cell wall, which may alter the ability of vancomycin to bind to its target (14, 15). Whether or not this cell wall thickening translates into altered virulence or is strain dependent has not been determined. A downregulation of agr, a quorum-sensing two-component regulatory system, has also been seen in some studies of MRSA VISA/hVISA; however, it is unclear whether agr dysfunction is similarly associated with MSSA RVS isolates (13, 16).
Most studies on the RVS phenotype have exclusively focused on MRSA cases; however, RVS remains relevant in MSSA cases because vancomycin is often used empirically in first-line therapy, and RVS may signal a bacterial cell wall alteration that affects clinical course (15, 17). The RVS phenotype in MSSA infection has recently been studied for its effect on clinical outcome. In particular, it has been linked to increased risk of mortality in some studies, although other analyses have failed to show robust associations with clinical outcome (15, 17–23).
However, the contribution of clonal lineage, regulatory system phenotypes, and other phenotypic characteristics has not yet been well defined in outcomes of invasive MSSA infection. Furthermore, the high genetic diversity among MSSA isolates (in contrast to the limited diversity in MRSA) has made it difficult to analyze links between clonal background and clinical outcomes (24). The RVS phenotype, in combination with genotypic information, may prove a more useful predictor of outcome. In this study, we sought to analyze the impact of the RVS phenotype, genetic lineage, and agr dysfunction on outcomes in MSSA bloodstream infections.
RESULTS
MSSA study cohort.
During the 2-year study period, 252 adult patients had bloodstream infections caused by MSSA. Of these, 84 patients (33%) were infected by MSSA isolates with vancomycin MICs ≥ 2 mg/liter (RVS phenotype), and 168 (67%) were infected with isolates with MICs < 2 mg/liter (non-RVS group). The majority of patients in the cohort were male (148/252 [59%]), and patients had an average age of 60.2 years (median, 60; interquartile range [IQR], 18 to 98; Table 1). The average Charlson comorbidity index (CCI) score was 5.4 (median, 5; range, 3 to 7), and 83% of patients (209/252) had a CCI of ≥3. The severity of illness at onset of bacteremia was low since patients had an average Pitt bacteremia score (PBS) of 2.3 (median, 2.3; IQR, 0 to 3), and 22% (56/252) had a score of ≥4. The most common primary sources of bacteremia were catheter-related infection (21%), pneumonia (16%), and skin-and-soft tissue infection (13%), while the source was unknown in 21% of cases. Overall, 42 (16%) patients developed endocarditis or deep endovascular infection, as determined by the clinical team and based on imaging studies (echocardiogram). Of the 53 catheter-related infections, 62% were central line-associated (33/53), and 38% were related to peripheral intravenous treatment (20/53). Surgical site infection (5%) and device-associated infection (3%) accounted for a small portion of the infections.
TABLE 1.
Baseline characteristics of BSI study population and bacterial isolates
| Variable | Total (n = 252) | No. (%) of patientsa with a vancomycin MIC of: |
Pb | |
|---|---|---|---|---|
| <2 mg/liter (n = 168) | 2 mg/liter (n = 84) | |||
| Demographics | ||||
| Female sex | 104 (41) | 72 (43) | 32 (38) | 0.47 |
| Mean age in yrs (IQR) | 60 (49–73) | 62 (52–75) | 56 (45–67) | 0.01* |
| Health care risk factors | ||||
| Admission from NH/LTCF | 30 (12) | 23 (14) | 7 (8) | 0.22 |
| Hemodialysis within past yr | 36 (14) | 23 (14) | 13 (15) | 0.70 |
| Hospital admission, <1 mo | 86 (34) | 56 (33) | 30 (36) | 0.71 |
| Hospital admission, <6 mo | 131 (52) | 89 (53) | 42 (50) | 0.66 |
| Surgery, <72 h | 15 (6) | 10 (6) | 5 (6) | 1 |
| Surgery, <1 yr | 71 (28) | 46 (27) | 25 (30) | 0.69 |
| Central venous catheter (temporary) | 34 (13) | 23 (14) | 11 (13) | 0.90 |
| Central venous catheter (permanent) | 43 (17) | 28 (17) | 15 (18) | 0.81 |
| Percutaneous device | 24 (10) | 15 (9) | 9 (11) | 0.65 |
| Primary site of infection by category | 0.47 | |||
| Bacteremia only | 53 (21) | 38 (23) | 15 (18) | |
| Catheter | 53 (21) | 34 (20) | 19 (23) | |
| Pneumonia | 40 (16) | 30 (18) | 10 (12) | |
| SSTI | 34 (13) | 23 (14) | 11 (13) | |
| Otherc | 72 (29) | 43 (26) | 29 (35) | |
| Secondary site of infection | 0.0011* | |||
| Metastatic or embolic | 59 (23) | 29 (17) | 30 (36) | |
| No secondary infection | 193 (77) | 139 (83) | 54 (64) | |
| Comorbidity and disease severity scores | ||||
| Mean Charlson comorbidity index (IQR) | 5.4 (3, 7) | 5.5 (4, 7) | 5.1 (2, 8) | 0.35 |
| Score ≥3 | 209 (83) | 148 (88) | 61 (73) | 0.002* |
| Mean Pitt bacteremia score (n = 246) (IQR) | 2.3 (0–3) | 2.2 (0–3) | 2.4 (0–4) | 0.12 |
| Score ≥4 | 56 (23) | 35 (21) | 21 (26) | 0.37 |
| Infection categoryd (n = 250) | ||||
| Hospital associated (HA) | 204 (82) | 136 (81) | 68 (83) | |
| Health care onset (HA/HO) | 138 (55) | 95 (57) | 43 (52) | 0.59 |
| Community onset (HA/CO) | 66 (26) | 41 (24) | 25 (30) | |
| Community associated (CA) | 46 (18) | 32 (19) | 14 (17) | |
| Treatment and clinical course | ||||
| Initial treatment (n = 245) | ||||
| Vancomycin only | 72 (29) | 43 (27) | 29 (35) | 0.20 |
| Vancomycin with other agent | 91 (37) | 56 (35) | 35 (42) | 0.29 |
| Beta-lactam with or without nonvancomycin agent | 67 (27) | 53 (33) | 14 (17) | 0.007* |
| Other antistaphylococcal agent | 10 (4) | 6 (4) | 4 (5) | 0.74 |
| Ineffective or absent therapy | 5 (2) | 3 (2) | 2 (2) | 1 |
| Treatment with ≥1 vancomycin dose (n = 245) | 177 (72) | 108 (67) | 69 (82) | 0.01* |
| Treatment alteration (n = 245) | ||||
| Altered within 2 days of initiation | 54 (22) | 34 (21) | 20 (24) | 0.63 |
| Altered within 7 days of initiation | 88 (36) | 53 (33) | 35 (42) | 0.18 |
| ID consult | 148 (59) | 91 (54) | 57 (68) | 0.04* |
| Mean bacteremia duration (IQR) | 2.3 (1–3) | 2.4 (1–3) | 2.1 (1–3) | 0.30 |
| >1 day | 95 (38) | 69 (41) | 26 (31) | 0.12 |
| >3 days | 48 (19) | 32 (19) | 16 (19) | 1 |
| >5 days | 20 (8) | 13 (8) | 7 (8) | 0.87 |
| All-cause mortality | ||||
| 30 day | 45 (18) | 31 (18) | 14 (17) | 0.73 |
| 60 day | 59 (23) | 41 (24) | 18 (21) | 0.60 |
| 90 day | 66 (26) | 47 (28) | 19 (23) | 0.36 |
| Death while bacteremic | 14 (6) | 11 (7) | 3 (4) | 0.40 |
| Strain characteristics | ||||
| Spa clonal complex | 0.02* | |||
| Spa-CC002 | 49 (19) | 27 (16) | 22 (26) | |
| Spa-CC008 | 36 (14) | 21 (13) | 15 (18) | |
| Spa-CC012 | 35 (14) | 30 (18) | 5 (6) | |
| Other | 132 (52) | 90 (54) | 42 (50) | |
| SpaCC, dichotomized | 0.01* | |||
| Spa-CC002 or Spa-CC008 | 85 (34) | 48 (29) | 37 (44) | |
| Other | 167 (66) | 120 (71) | 47 (56) | |
| agr deficient | 20 (8) | 12 (7) | 8 (10) | 0.51 |
| Antibiotic nonsusceptibility | ||||
| Fluoroquinolone (levofloxacin) | 30 (12) | 18 (11) | 12 (14) | 0.41 |
| Macrolides (erythromycin) | 93 (37) | 68 (40) | 25 (30) | 0.10 |
| Tetracycline | 14 (6) | 6 (4) | 8 (10) | 0.07 |
| Penicillin | 157 (83) | 99 (59) | 58 (69) | 0.54 |
| Bactrim | 9 (4) | 6 (4) | 3 (4) | 1 |
Except as indicated otherwise in column 1.
*, Statistically significant (P < 0.05) as determined by chi-square, Fisher exact, two-sample t, or Wilcoxon rank sum test, where appropriate.
“Other” includes UTI, device, surgical site, endovascular, CNS, and osteoarticular primary sites.
Comparison of HA/CO versus HA/HO versus CA infection categories.
The majority of patients had health care-associated infections (n = 204, 82%) with 26% (n = 66) being community-onset infections [HA-CO] and 55% (n = 138) hospital-onset infections ([HA-HO]). Thirty patients (12%) were admitted directly from a long-term care facility, 14% (n = 36) were receiving hemodialysis, 52% (n = 131) had been hospitalized in the past 6 months, and 34% (n = 86) had been hospitalized in the past 1 month. At the time of bacteremia, 13% (n = 34) had a temporary central venous catheter (CVC), and 17% (n = 43) had a permanent CVC in place. 10% (n = 24) had percutaneous devices, and 6% (n = 15) underwent surgery in the 72 h preceding bacteremia. Only 18% (n = 46) of infections were community associated (CA).
The 30-day mortality for the cohort was 18% (n = 45), and the 90-day mortality was 26% (n = 66). The average duration of bloodstream infection was 2.3 days (median, 1 day; IQR, 1 to 3 days), and 19% (n = 48) of the patients had a bloodstream infection >3 days in length. A total of 6% (n = 14) of the patients died while still actively bacteremic. Endovascular infection was a complication of 17% (n = 42) of bloodstream infections, and metastatic or embolic spread of infection was documented in 23% of the cases overall (n = 59).
Molecular and phenotypic characterization of MSSA strains.
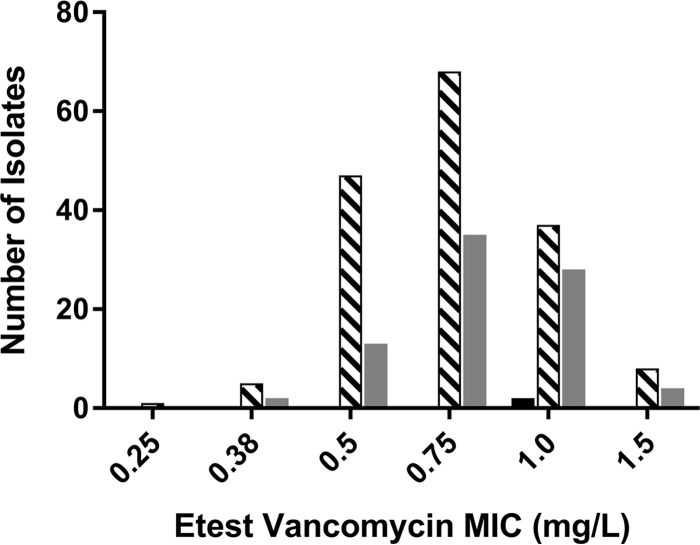
The collection of BSI isolates was highly genetically diverse: 127 spa types were identified among 252 clinical isolates. A total of 32 isolates (13%) were spa type t002, and 16 isolates were spa type t008 (6%). Another 100 isolates (40%) had spa types that occurred only once. Spa-CC002 was the most prevalent clonal cluster (CC), or group of related strain types, with 49 isolates (19%). Spa-CC008 and Spa-CC012 each accounted for 14% (n = 36 and n = 35, respectively). Regarding antibiotic nonsusceptibility, 12% (n = 30) of the isolates were resistant to fluoroquinolones, 37% (n = 93) to were resistant macrolides, 6% (n = 14) were resistant to tetracyclines, 83% (n = 157) were resistant to penicillin, and 4% (n = 9) were resistant to trimethoprim-sulfamethoxazole. A small proportion (n = 20, 8%) of isolates were agr deficient. We also carried out vancomycin Etests on 99% (250/252) of our isolates to ascertain the RVS phenotype by an alternative method. We found that a majority (n = 194 [78%]) were in agreement, having automated MIC values that were ≤1 dilution different from the Etest values (Fig. 1). In addition, applying the Spearman correlation test showed that the Etest and Microscan results correlated (Spearman correlation coefficient = 0.126, P = 0.046). Of note, all 56 isolates that were not in agreement had higher Microscan MICs than Etest MICs. The study was not powered to assess the Etest-defined RVS phenotype as the exposure variable of interest.
FIG 1.
Comparison of Etest and Microscan MICs. Microscan vancomycin MIC: ■, 0.5 mg/liter; ▧, 1.0 mg/liter; ▩, 2.0 mg/liter.
Predictors of RVS phenotype.
Univariable analyses comparing the groups infected by Microscan RVS and non-RVS phenotype isolates showed that patients in the RVS group were significantly younger (average age, 56 years versus 62 years; P = 0.01). However, there was no significant difference in gender distribution between the RVS and non-RVS groups (Table 1). Patients in the two groups had similar frequencies of health care-associated exposures, and there was no significant difference in the severity of illness at bacteremia onset, as assessed by the PBS (average PBS, 2.2 versus 2.4; P = 0.12). Although the average CCI scores were similar, fewer patients in the RVS group had a CCI ≥ 3 (73% versus 88%; P = 0.0002). There was a similar frequency of sources of infection across the groups, but significantly more patients in the RVS phenotype group developed endovascular infection (26% versus 12%; P = 0.004). Furthermore, a greater proportion of patients in the RVS group had metastatic seeding or embolic spread of (36% versus 17%; P = 0.001). Significantly more isolates from the Spa-CC002 or Spa-CC008 lineages were found in the RVS phenotype group (44% versus 29%; P = 0.01) compared to isolates from the non-CC002/CC008 clonal backgrounds. There were no significant differences in antibiotic susceptibility patterns between RVS and non-RVS groups.
Complications of MSSA bacteremia and RVS phenotype.
In unadjusted analyses of 30-day mortality, we found no difference in frequency of the RVS phenotype between groups (Table 2). Significant predictors of 30-day mortality included age ≥ 65 years (56% versus 32%; P = 0.003), hospital admission within the past 1 month (49% versus 31%; P = 0.02), a Pitt bacteremia score ≥ 4 (57% versus 15%; P < 0.0001), and pneumonia as a primary source of bacteremia (27% versus 14%; P = 0.03; Table 2). Hemodialysis was protective in unadjusted analysis (2% versus 17%; P = 0.03). In a multivariable analysis of 30-day mortality predictors, only hospital admission within the past 1 month remained a significant predictor of mortality (adjusted odds ratio [aOR], 2.12 [1.06 to 4.24]; P = 0.03).
TABLE 2.
Predictors of 30-day mortality
| Variable | 30-day mortality |
||
|---|---|---|---|
| No. (%) of patientsa |
P | ||
| No (n = 207) | Yes (n = 45) | ||
| Demographics | |||
| Female sex | 80 (39) | 24 (53) | 0.07 |
| Age >65 yrs | 66 (32) | 25 (56) | 0.003b |
| Health care risk factors | |||
| Admission from NH/LTCF | 22 (11) | 8 (18) | 0.18 |
| Hemodialysis, <1 yr | 35 (17) | 1 (2) | 0.01b |
| Hospital admission, <1 mo | 64 (31) | 22 (49) | 0.02b |
| Hospital admission, <6 mo | 108 (52) | 23 (51) | 0.90 |
| Hospital associatedc (n = 250) | 166 (81) | 38 (85) | |
| Health care onset (HA/HO) | 117 (57) | 21 (47) | 0.16 |
| Community onset (HA/CO) | 49 (24) | 17 (38) | |
| Community associated (CA) | 39 (19) | 7 (16) | |
| Surgery, <72 h | 12 (6) | 3 (7) | 0.73 |
| Surgery, <1 year | 61 (29) | 10 (22) | 0.33 |
| Central venous catheter (temporary) | 25 (12) | 9 (20) | 0.16 |
| Central venous catheter (permanent) | 38 (18) | 5 (11) | 0.07b |
| Percutaneous device | 20 (10) | 4 (9) | 1.0 |
| Primary site of infection (categorized) | 0.061 | ||
| Bacteremia only | 41 (20) | 12 (27) | |
| Catheter associated | 48 (23) | 5 (11) | |
| Pneumonia | 28 (14) | 12 (27) | |
| SSTI | 27 (13) | 7 (16) | |
| Otherd | 63 (30) | 9 (19) | |
| Secondary site of infection | 0.34 | ||
| Metastatic or embolic | 46 (22) | 13 (29) | |
| No secondary infection | 161 (78) | 32 (71) | |
| Mean comorbidity and disease severity scores | |||
| Mean Charlson comorbidity index (IQR) | 5.2 (3–7) | 6.0 (5–7) | 0.10 |
| Score ≥3 | 167 (81) | 42 (93) | 0.04b |
| Mean Pitt bacteremia score (n = 246) (IQR) | 1.6 (0–2) | 4.5 (2–9) | <0.001b |
| Score ≥4 | 31 (15) | 25 (57) | <0.001b |
| Treatment and clinical course | |||
| Initial treatment (n = 245) | |||
| Vancomycin only | 61 (31) | 11 (24) | 0.42 |
| Vancomycin with other agent | 70 (35) | 21 (47) | 0.14 |
| Beta-lactam with or without nonvancomycin agent | 56 (28) | 11 (24) | 0.62 |
| Other antistaphylococcal agent | 8 (4) | 2 (4) | 1.0 |
| Ineffective or absent therapy | 5 (3) | 0 (0) | 0.59 |
| Treated with ≥1 vancomycin dose (n = 245) | 140 (70) | 37 (82) | 0.10 |
| Treatment alteration (n = 245) | |||
| Altered within 2 days of initiation | 46 (23) | 8 (18) | 0.44 |
| Altered within 7 days of initiation | 74 (37) | 14 (31) | 0.46 |
| ID consult | 120 (60) | 24 (53) | 0.41 |
| Mean bacteremia duration (IQR) | |||
| >1 day | 77 (37) | 18 (40) | 0.73 |
| >3 days | 39 (19) | 9 (20) | 0.86 |
| >5 days | 14 (7) | 6 (13) | 0.14 |
| Strain characteristics | |||
| SpaCC, dichotomized | 0.27 | ||
| Spa-CC002 or spa-CC008 | 73 (35) | 12 (27) | |
| Other | 134 (65) | 33 (73) | |
| agr deficient | 18 (9) | 2 (4) | 0.54 |
| Vancomycin MIC, 2 mg/liter | 70 (34) | 14 (31) | 0.72 |
Except as indicated otherwise in column 1.
Statistically significant (P < 0.05) as determined by chi-square, Fisher exact, two sample t test, or Wilcoxon rank sum tests, where appropriate.
Comparison of HA/CO versus HA/HO versus CA infection categories.
“Other” includes UTI, device, surgical site, endovascular, CNS, and osteoarticular primary sites.
Complicated bacteremia, as defined by metastatic or embolic seeding, endovascular infection, or duration >3 days, was evaluated in univariable and multivariable models. In unadjusted analysis, there were significantly more isolates with vancomycin MIC of ≥2 mg/liter in the complicated bacteremia group (43% versus 29%; P = 0.04). The multivariable model included the variables of age, gender, and any variables from the univariable analysis with a P value ≤ 0.2 (Table 3). A vancomycin MIC of ≥2 mg/liter remained a significant predictor of complicated infection in the multivariable model (aOR, 2.35 [1.26 to 4.37]; P = 0.007). A Charlson score of ≥3 was also a significant predictor of complicated infection (aOR, 2.63 [1.09 to 6.37]; P = 0.03) in this model. There were no significant differences in genotype (as dichotomized into Spa-CC002/CC008 versus others) between groups in either univariable or adjusted analyses (Table 3).
TABLE 3.
Predictors of complicated bacteremiaa
| Variable | No. (%) of patientsb |
Univariable P value | aOR (95% CI)c | Multivariable P value | |
|---|---|---|---|---|---|
| No (n = 172) | Yes (n = 80) | ||||
| Vancomycin MIC (2 mg/liter) | 50 (29) | 34 (43) | 0.04 | 2.35 (1.26–4.37) | 0.007 |
| Spa-CC (dichotomized) | 0.15 | 0.08 | |||
| Spa-CC002 or Spa-CC008 | 63 (37) | 22 (28) | 0.56 (0.30–1.06) | ||
| Other Spa-CC | 109 (63) | 58 (73) | REF | ||
| Race | 0.04 | 0.03 | |||
| White | 43 (25) | 29 (36) | 1.07 (0.48–2.37) | 0.87 | |
| African-American | 40 (23) | 9 (11) | 0.29 (0.11–0.78) | 0.01 | |
| Hispanic | 57 (33) | 23 (29) | 0.61 (0.27–1.36) | 0.22 | |
| Other | 29 (17) | 19 (24) | REF | ||
| Hemodialysis, <1 yr | 20 (12) | 16 (20) | 0.08 | 2.02 (0.95–4.43) | 0.08 |
| Hospital admission, <1 yr | 98 (57) | 53 (66) | 0.16 | 1.49 (0.82–2.70) | 0.19 |
| Percutaneous device | 20 (12) | 4 (5) | 0.10 | 0.29 (0.09–0.94) | 0.04 |
| Charlson score ≥3 | 138 (80) | 71 (89) | 0.09 | 2.63 (1.09–6.37) | 0.03 |
Except as indicated otherwise in column 1.
That is, metastatic, embolic, or endovascular infection for >3 days.
aOR, adjusted odds ratio; REF, reference category; CI, confidence interval.
DISCUSSION
In this retrospective observational study of MSSA bloodstream infections we found increased rates of complicated bacteremia, as defined by metastatic spread of disease, endovascular infection or duration >3 days, in the MSSA BSIs caused by isolates exhibiting the RVS phenotype. However, we did not observe a significant effect of a high vancomycin MIC on all-cause 30-day mortality.
Our data show high overall mortality rates in MSSA BSI, consistent with those seen in prior studies (5, 6, 25). The persistence of these high mortality rates and other adverse outcomes, even in the presence of effective antimicrobial therapy, highlights the urgent need to better understand prognostic and modifiable risk factors for poor outcome in MSSA BSI. We controlled for comorbidity burden and severity of disease in our analysis. While our study was retrospective in nature, our clinical data were rich, and we additionally evaluated the role of genotypic background in clinical outcomes. There have been few studies on the relationship between vancomycin MIC and mortality in MSSA BSI that incorporate both genotype and agr dysfunction into the assessment of MIC effect.
Regarding mortality effect, some prior studies on the relationship between vancomycin MIC and outcomes in MSSA BSI have found an increased mortality risk (19, 20) associated with MICs ≥ 2 mg/liter, however other recent studies have failed to demonstrate a statistically significant difference in death rates (2, 23, 26). A meta-analysis looking at effect of vancomycin MIC on mortality in S. aureus bacteremia failed to show a significant difference in 30-day mortality rates in SAB across a range of vancomycin MIC cutoffs (21). Most of the studies in this meta-analysis focused on MRSA, as only a limited number of prior studies have examined the predictive value of vancomycin MIC in MSSA SAB. The variation in results may be due to lack of power, differences in clinical features of the study populations, failure to control for confounding factors such as comorbidity burden, and varying MIC measurement methodologies (automated versus nonautomated) that may alter the MIC groupings. It also may be that the effect of MIC on short-term mortality in MSSA BSI is minimal but the reduced susceptibility phenotype is a marker of virulence that drives morbidity and complications such as endocarditis.
Our findings in unadjusted analyses found that the RVS phenotype was associated with younger age, lower comorbidity burden, metastatic or embolic spread of infection, and endovascular infection and suggest that a vancomycin MIC ≥ 2 mg/liter may be a marker for heightened virulence. The association between MIC ≥ 2 mg/liter and complicated infection remained significant in adjusted analyses. Similarly, a different study of catheter-related MSSA bloodstream infections found that an MIC ≥ 1.5 mg/liter by Etest was associated with complicated MSSA BSI, defined by the presence of either endocarditis, septic thrombophlebitis, metastatic or hematogenous spread, persistent bacteremia, or persistent fever (2). Another study of MSSA BSI found increased rates of septic thrombophlebitis with vancomycin MIC ≥ 1.5 mg/liter (Etest) but failed to show differences in risk for mortality, endocarditis or hematogenous seeding (26). This study, however, was limited by small cohort size and small number of isolates with MIC ≥ 1.5 mg/liter that were included in the analysis. If pathogenicity is attributable to heightened MIC, it is still unclear what exact mechanism is responsible for this relationship. Different explanations ranging from impaired therapeutic efficacy in the setting of a thickened cell wall to metabolic changes or changes in virulence factor expression have been proposed (17, 27).
It is also possible that the increased MIC is a marker for other mediating factors, such as clonal background or broader antibiotic resistance. We did not observe any significant differences in susceptibilities to other antibiotic classes for RVS versus non-RVS isolates. However, we found differences in frequency of the RVS phenotype among clonal backgrounds. Despite the high genetic diversity among MSSA isolates in our study, the RVS phenotype was significantly overrepresented in Spa-CC002 and Spa-CC008 strains versus non-CC002/CC008 strains. Most CC002 and CC008 strains belong to the ST5 and ST8 lineages, respectively (28). Along similar lines, in a prior examination of candidate gene loci of VISA and hVISA MRSA isolates, we found a greater number of polymorphisms in genes that contribute to mechanisms of intermediate vancomycin susceptibility in ST5 and ST8 strains (29), suggesting a link between susceptibility phenotype and clonal lineage. The exact nature of this association cannot be fully elucidated based on sequencing of the spa locus alone. Both bacterial genome-wide association studies (GWAS) and whole-genome sequencing (WGS) approaches would help to identify contributions of clone-specific polymorphisms to the RVS phenotype.
We chose to use vancomycin MICs derived from automated methods (Microscan) in our study, since such methods are the ones more often used in clinical practice and therefore inform real-time clinical decision-making. Particularly in the case of MSSA infection, nonautomated methods such as Etest or broth microdilution (BMD) are rarely used in the clinical setting. The use of MICs generated by automated methods limits full comparison with prior studies that have used nonautomated methods such as Etest or BMD (2, 19, 20). Studies comparing methodologies show that, depending on the standard to which compared, automated methods such as Microscan may overestimate, approximate, or underestimate the true MIC values (30–33). Therefore, use of an automated method may produce a different categorization with regard to this exposure variable, in part because of differences in the concentrations assayed. When we compared Etests to Microscan, we found the Etest and Microscan results were in essential agreement in a majority of cases, with MIcroScan overall yielding higher MIC values. While automated methods such as Microscan do not perfectly replicate results from clinical standards, due to their widespread use they are of important clinical relevance and thus were the focus of our analysis.
Several other limitations in our study deserve consideration. First, it occurred at a single institution, potentially limiting the generalizability of our findings (2, 18–20). Second, the available clinical data did not include granular information on treatment (e.g., duration or therapy adjustments), limiting our ability to control for this important determinant of patient outcomes. Furthermore, while we had access to information on hospital courses within our medical system, we did not have access to information regarding whether or not patients had outside hospital admissions that may have captured further morbidity and mortality. Third, the high genetic diversity of our MSSA collection decreased our power to detect meaningful differences in clinical outcomes based on clonal lineage. We were also underpowered to assess the impact of agr deficiency on morbidity and mortality.
In summary, while we found no association between the RVS phenotype and mortality in our study, we identified an important association with complicated infection. We also found an association between the phenotype and specific clonal background, younger age and lower burden of comorbidity. Taken together, these findings suggest the RVS phenotype as a potential marker of strain virulence. Further studies need to be conducted to explore this host-pathogen relationship.
MATERIALS AND METHODS
Study population.
We carried out a retrospective, observational cohort study of all patients ≥ 18 years of age with a blood culture positive for MSSA from 1 January 2010 through 1 January 2012. The study was approved by the Institutional Review Board. Patients infected with MSSA BSIs were identified via blood culture logs maintained by the clinical microbiology laboratory. This academic tertiary care medical center is comprised of a large adult inpatient hospital, as well as a community hospital that is a referral center for local nursing facilities. Patients were excluded if medical charts were not available, the first identifiable culture was postmortem, or the culture was from a sterile body fluid other than blood.
An infectious diseases physician was responsible for reviewing all chart information. We extracted demographic and clinical information from the patient's charts, including prior hospital admissions, transfer from a nursing home/long-term-care facility, dialysis, invasive surgery, presence of a central venous catheter, and presence of a percutaneous device. The bloodstream infections were categorized as community onset (CO) if the initial culture was collected within 72 h of admission and hospital-onset (HO) if collected greater than 72 h after admission. Data on primary site of infection were collected (34). The primary site of infection was defined based on reported symptoms prior to bacteremia, coupled with diagnostic studies. Metastatic infections were defined as additional sites of infections that developed with a temporal delay after the onset of bacteremia or when the clinical evidence (i.e., multiple septic emboli on central nervous system [CNS] imaging) clearly supported metastatic or embolic disease. In cases where the site of infection was not clearly stated in the chart, the source was inferred from available clinical indicators or, if none were identifiable, it was categorized as an “unknown source.” Data on patient comorbidities were collected to assign a Charlson comorbidity index (CCI) score to each patient (35). The Pitt bacteremia score (PBS) was also used to assess the severity of illness at time of the incident blood culture (36, 37). Both scores were dichotomized based on previously investigated cutoffs that are predictive of mortality (14). Only the first bacteremia episode during the study period was included in this analysis. The main outcome was all-cause 30-day mortality. The secondary outcome was complicated bacteremia, defined by metastatic or embolic seeding, endovascular infection, or a duration of >3 days.
Microbiological and molecular studies.
Vancomycin and all other antibiotic MICs were determined by automated Microscan with Prompt (Beckman Coulter) inoculation according to Clinical and Laboratory Standards Institute (CLSI) standards (38). Vancomycin Etests (bioMérieux) were performed on 99% (250/252) of isolates according to the manufacturer's instructions. The vancomycin MIC was dichotomized, with the RVS phenotype defined as an MIC of ≥2 mg/liter (Microscan).
agr dysfunction was detected via the standard delta-hemolysin production assay (strains negative for delta-hemolysin production are considered agr deficient) (39). The repeat region of the staphylococcal protein A (spa) gene was sequenced, assigned a spa type, and clustered using the BURP (based upon repeat pattern) algorithm via Ridom StaphType software (v2.2.1) as previously described (40, 41).
Statistical analyses.
All statistical analyses were completed using SAS v9.4 (SAS Institute, Inc.). Agreement between the Microscan and Etest measurements of vancomycin MIC was measured using the nonparametric Spearman correlation coefficient. Univariable relationships of strain and patient characteristics with the RVS phenotype were tested using chi-squared, Fisher exact, Student t test, or Wilcoxon rank sum tests where appropriate. Similar tests were used to investigate the relationship of clinical factors and strain characteristics to the primary and secondary outcomes, all-cause mortality and complicated bacteremia, respectively. These relationships were further assessed using multivariable logistic regression models. The RVS phenotype, gender, and age were included in the final model a priori. Other demographic and known risk factors were selected into the model if the univariable P value of <0.20. After assessing multicollinearity of variables, the most parsimonious model was selected.
Logistic regression models were used to test the hypothesized interaction between the RVS phenotype on mortality. To examine how clonal background modifies the effect of RVS phenotype on outcome, we included an interaction term. Similarly, interaction between either the RVS phenotype or clonal lineage and the infection category (i.e., health care associated/hospital onset, health care associated/community onset, and community associated) was tested using multivariable logistic regression models. The interaction terms were evaluated using the Wald chi-square test. We also hypothesized that PBS (as a proxy for disease severity) or embolic/metastatic spread would mediate the relationship between clonal lineage and outcome. Since there was no significant association between dichotomized PBS or embolic/metastatic spread and outcome, the requirements for mediation analysis were not met.
ACKNOWLEDGMENTS
This study was in part funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant K08 AI090013 to A.-C.U.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Aguado JM, San-Juan R, Lalueza A, Sanz F, Rodriguez-Otero J, Gomez-Gonzalez C, Chaves F. 2011. High vancomycin MIC and complicated methicillin-susceptible Staphylococcus aureus bacteremia. Emerg Infect Dis 17:1099–1102. doi: 10.3201/eid1706.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatib R, Sharma M, Iyer S, Fakih MG, Obeid KM, Venugopal A, Fishbain J, Johnson LB, Segireddy M, Jose J, Riederer K. 2013. Decreasing incidence of Staphylococcus aureus bacteremia over 9 years: greatest decline in community-associated methicillin-susceptible and hospital-acquired methicillin-resistant isolates. Am J Infect Control 41:210–213. doi: 10.1016/j.ajic.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, Coombs GW, Murray RJ, Howden B, Johnson PD, Dowling K; Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis. 2009. Staphylococcus aureus bacteremia: a major cause of mortality in Australia and New Zealand. Med J Aust 191:368–373. [DOI] [PubMed] [Google Scholar]
- 5.Shurland S, Zhan M, Bradham DD, Roghmann MC. 2007. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 28:273–279. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 6.Mylotte JM, McDermott C, Spooner JA. 1987. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis 9:891–907. doi: 10.1093/clinids/9.5.891. [DOI] [PubMed] [Google Scholar]
- 7.David MZ, Daum RS, Bayer AS, Chambers HF, Fowler VG Jr, Miller LG, Ostrowsky B, Baesa A, Boyle-Vavra S, Eells SJ, Garcia-Houchins S, Gialanella P, Macias-Gil R, Rude TH, Ruffin F, Sieth JJ, Volinski J, Spellberg B. 2014. Staphylococcus aureus bacteremia at 5 US academic medical centers, 2008-2011: significant geographic variation in community-onset infections. Clin Infect Dis 59:798–807. doi: 10.1093/cid/ciu410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laupland KB, Lyytikainen O, Sogaard M, Kennedy KJ, Knudsen JD, Ostergaard C, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schonheyder HC, International Bacteremia Surveillance C . 2013. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 19:465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 9.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 36:1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 10.McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP Jr, Miller RR, Furuno JP. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 45:329–337. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]
- 11.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Sakoulas G, Perencevich EN. 2010. Empiric antibiotic therapy for Staphylococcus aureus bacteremia may not reduce in-hospital mortality: a retrospective cohort study. PLoS One 5:e11432. doi: 10.1371/journal.pone.0011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardete S, Tomasz A. 2014. Mechanisms of vancomycin resistance in Staphylococcus aureus. J Clin Invest 124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howden BP, Peleg AY, Stinear TP. 2014. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect Genet Evol 21:575–582. doi: 10.1016/j.meegid.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Iwamoto A, Lian JQ, Neoh HM, Maruyama T, Horikawa Y, Hiramatsu K. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 50:428–438. doi: 10.1128/AAC.50.2.428-438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardete S, Kim C, Hartmann BM, Mwangi M, Roux CM, Dunman PM, Chambers HF, Tomasz A. 2012. Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLoS Pathog 8:e1002505. doi: 10.1371/journal.ppat.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caston JJ, Gonzalez-Gasca F, Porras L, Illescas S, Romero MD, Gijon J. 2014. High vancomycin minimum inhibitory concentration is associated with poor outcome in patients with methicillin-susceptible Staphylococcus aureus bacteremia regardless of treatment. Scand J Infect Dis 46:783–786. doi: 10.3109/00365548.2014.931596. [DOI] [PubMed] [Google Scholar]
- 19.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Gao W, Christiansen KJ, Coombs GW, Johnson PD, Howden BP. 2011. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 204:340–347. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 20.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Johnson PD, Howden BP. 2013. Vancomycin minimum inhibitory concentration, host comorbidities and mortality in Staphylococcus aureus bacteraemia. Clin Microbiol Infect 19:1163–1168. doi: 10.1111/1469-0691.12168. [DOI] [PubMed] [Google Scholar]
- 21.Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. 2014. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564. doi: 10.1001/jama.2014.6364. [DOI] [PubMed] [Google Scholar]
- 22.Takesue Y, Nakajima K, Takahashi Y, Ichiki K, Ishihara M, Wada Y, Tsuchida T, Uchino M, Ikeuchi H. 2011. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 mug/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J Infect Chemother 17:52–57. doi: 10.1007/s10156-010-0086-0. [DOI] [PubMed] [Google Scholar]
- 23.Baxi SM, Clemenzi-Allen A, Gahbauer A, Deck D, Imp B, Vittinghoff E, Chambers HF, Doernberg S. 2016. Vancomycin MIC does not predict 90-day mortality, readmission, or recurrence in a prospective cohort of adults with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 60:5276–5284. doi: 10.1128/AAC.00658-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goering RV, Shawar RM, Scangarella NE, O'Hara FP, Amrine-Madsen H, West JM, Dalessandro M, Becker JA, Walsh SL, Miller LA, van Horn SF, Thomas ES, Twynholm ME. 2008. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from global clinical trials. J Clin Microbiol 46:2842–2847. doi: 10.1128/JCM.00521-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Cortes LE, Velasco C, Retamar P, del Toro MD, Galvez-Acebal J, de Cueto M, Garcia-Luque I, Caballero FJ, Pascual A, Rodriguez-Bano J. 2015. Is reduced vancomycin susceptibility a factor associated with poor prognosis in MSSA bacteraemia? J Antimicrob Chemother 70:2652–2660. doi: 10.1093/jac/dkv133. [DOI] [PubMed] [Google Scholar]
- 27.Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. 2009. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 49:1169–1174. doi: 10.1086/605636. [DOI] [PubMed] [Google Scholar]
- 28.Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW, European Staphylococcal Reference Laboratory Working G. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med 7:e1000215. doi: 10.1371/journal.pmed.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafer C, Lin Y, Kornblum J, Lowy FD, Uhlemann AC. 2012. Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 56:5845–5851. doi: 10.1128/AAC.01139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland CM, Porr WH, Davis KA, Mansell KB. 2010. Vancomycin MIC susceptibility testing of methicillin-susceptible and methicillin-resistant Staphylococcus aureus isolates: a comparison between Etest® and an automated testing method. South Med J 103:1124–1128. doi: 10.1097/SMJ.0b013e3181efb5b1. [DOI] [PubMed] [Google Scholar]
- 31.Chen SY, Liao CH, Wang JL, Chiang WC, Lai MS, Chie WC, Chang SC, Hsueh PR. 2014. Method-specific performance of vancomycin MIC susceptibility tests in predicting mortality of patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 69:211–218. doi: 10.1093/jac/dkt340. [DOI] [PubMed] [Google Scholar]
- 32.Swenson JM, Anderson KF, Lonsway DR, Thompson A, McAllister SK, Limbago BM, Carey RB, Tenover FC, Patel JB. 2009. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. J Clin Microbiol 47:2013–2017. doi: 10.1128/JCM.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadarajah R, Post LR, Liu C, Miller SA, Sahm DF, Brooks GF. 2010. Detection of vancomycin-intermediate Staphylococcus aureus with the updated Trek-Sensititre System and the MicroScan System: comparison with results from the conventional Etest and CLSI standardized MIC methods. Am J Clin Pathol 133:844–848. doi: 10.1309/AJCPMV1P0VKUAZRD. [DOI] [PubMed] [Google Scholar]
- 34.Austin ED, Sullivan SB, Whittier S, Lowy FD, Uhlemann AC. 2016. Peripheral intravenous catheter placement is an underrecognized source of Staphylococcus aureus bloodstream infection. Open Forum Infect Dis 3:ofw072. doi: 10.1093/ofid/ofw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 37.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement; M100–S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology 154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37:3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]