FIG 2 .
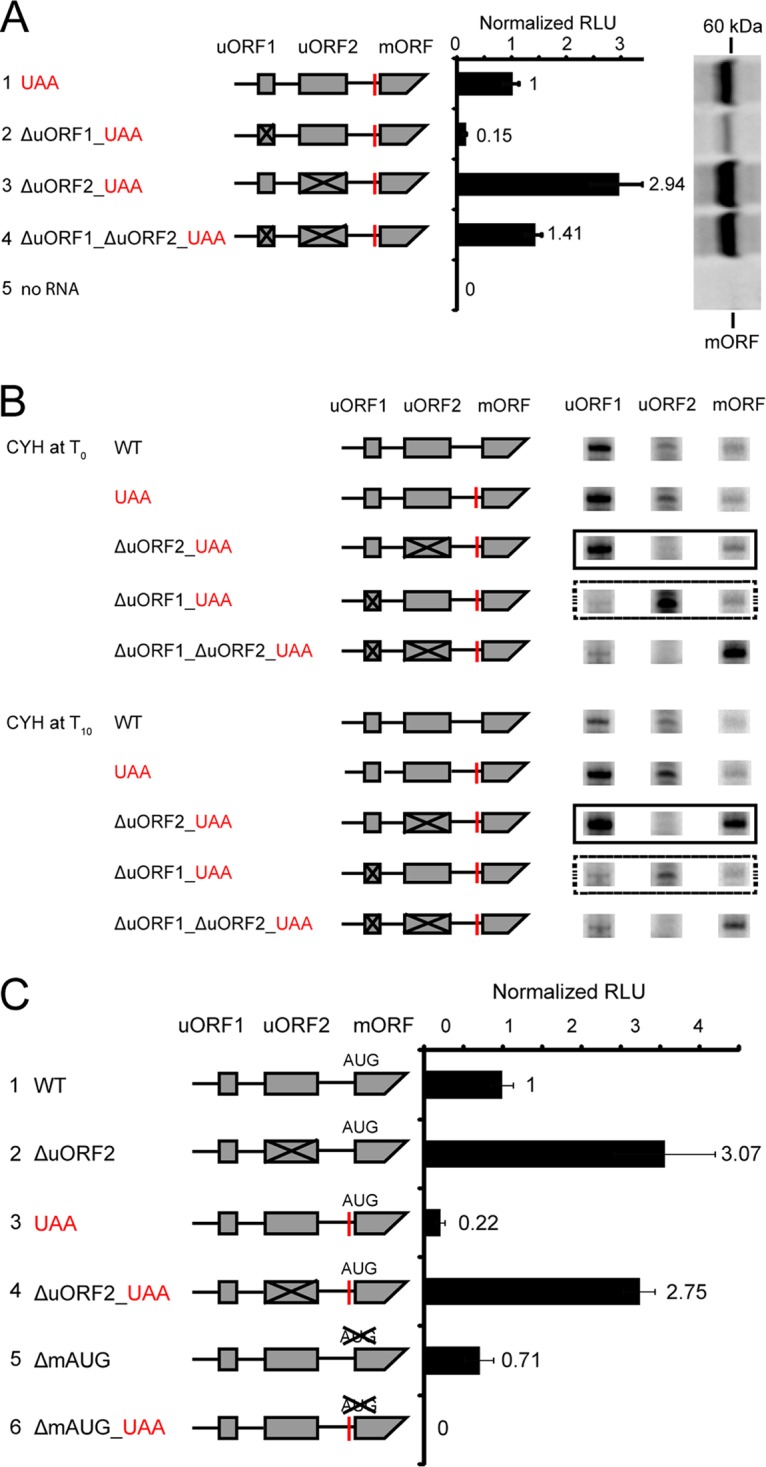
Contribution of cpc-1 uORF1 and uORF2 to the regulation of translation from the mAUG in N. crassa cell extracts. (A) Effects of eliminating cpc-1 uORF1 and uORF2 on translation from the mAUG. Constructs (numbered 1 to 4) contained the UAA stop codon (red bar) to eliminate translation from upstream in-frame NCCs and the indicated mutations to uORF start codons to eliminate initiation from them (uORF1 AUG to AAA and/or uORF2 AUG to ACA). Capped and polyadenylated mRNA (6 ng) was used to program N. crassa translation reaction mixtures (10 µl). LUC activity produced from mRNAs 2 to 4 obtained after 30 min of incubation at 26°C was calculated relative to the activity produced from mRNA 1. Mean values and standard deviations from three independent experiments, each performed in triplicate, are given as normalized relative light units (RLU). In addition, [35S]Met-labeled translation products from translation reactions programmed with mRNAs 1 to 4 or with no mRNA were analyzed on 12% NuPAGE gels, and a representative gel is shown. The position of radiolabeled LUC produced from the mAUG is indicated. (B) Toeprint analysis indicates reinitiation following translation of cpc-1 uORF1 but not uORF2. cpc-1–luc mRNA (60 ng) was used to program 20-µl N. crassa cell-free translation reaction mixtures. WT mRNA containing the wild-type cpc-1 5′ leader and the mRNAs used in panel A were analyzed in parallel along with controls. Reaction mixtures were incubated at 26°C min with cycloheximide (CYH) added either prior to incubation (T0) or after 10 min of incubation (T10) as indicated. Radiolabeled primer CPC101 was used to examine ribosomes at uORF1 and uORF2; primer ZW4 was used to examine ribosomes at the mORF. The original data from which the toeprint signals were excised are shown in Fig. S7. (C) Discriminating translation from N. crassa cpc-1 NCCs and mAUG in vitro. Capped and polyadenylated mRNA (6 ng) was used to program N. crassa translation reaction mixtures (10 µl) with the indicated constructs. Firefly luciferase activity from each mRNA obtained after 30 min of incubation at 26°C was calculated relative to synthesis from the WT construct. Mean values and standard deviations from three independent experiments, each performed in triplicate, are plotted.
