Fig. 2.
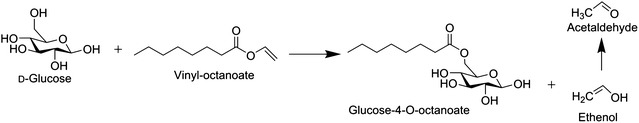
Transesterification between glucose and vinyl-octanoate which leads to the formation of glucose–4–O–octanoate and ethenol. Ethenol is not stable and tautomerizes to acetaldehyde. Acetaldehyde evaporates quickly, pushing the reaction forward. The reaction scheme is analogous for other monosaccharides like xylose. It might be possible that more or other C-atoms are acylated, too. Using a fatty acid like octanoic acid, one molecule of water will be formed as a side product
