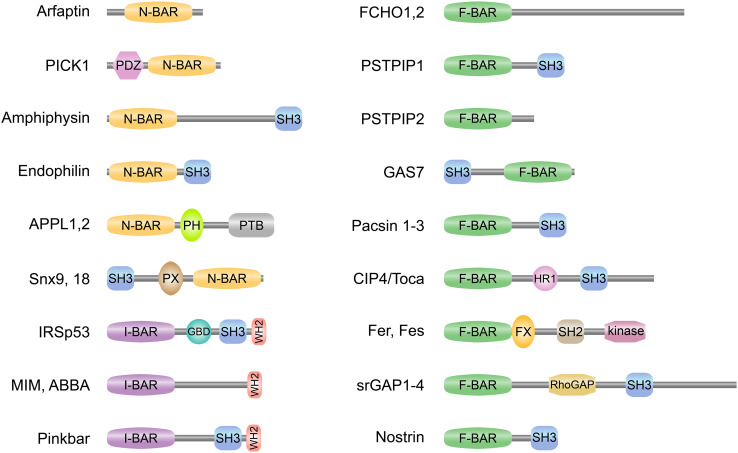
Fig. 2.
Schematic domain representation of selected BAR domain proteins. Selected members of the N-BAR (BAR with an N-terminal amphipathic helix) and I-BAR (inverse-BAR) domain family on left and F-BAR (Fes/CIP4 homology-BAR) domain family on right are depicted. Most BAR domain proteins contain one or several additional domains with lipid-binding, protein-binding, and/or enzymatic activities. PDZ (PSD95/Dlg1/ZO-1) domain mediates protein–protein interactions by binding to the C-terminus of other specific proteins. SH3 (Src homology 3) domain confers binding to poly-proline motifs of target proteins, like N-WASP or dynamin. The phosphoinositides-binding PX (phox homology) and PH (pleckstrin homology) domains modulate membrane-binding specificities of different subsets of N-BAR domain proteins. PTB (phosphotyrosine-binding) domain binds to phosphotyrosine. GBD (GTPase-binding domain) is required for binding to Rho small GTPases. WH2 (Wiskott-Aldrich syndrome homology 2) domain binds to actin monomers and can facilitate the assembly of actin monomers into actin filaments. HR1 (protein kinase C-related kinase homology region 1) binds the small G protein Rho. FX (F-BAR extension) domain in Fer was shown to bind phosphatidic acid. SH2 (Src homology 2) domain allows binding to phosphorylated tyrosine residues on other proteins. RhoGAP (Rho GTPase activating protein) domain modulates the activity of Rho. Fer and Fes possess a tyrosine kinase domain