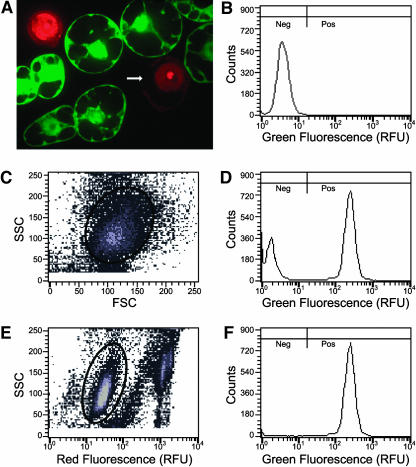
Figure 2.
Propidium Iodide Staining of Protoplast Preparations Improves Flow Cytometry Resolution.
(A) A 1-μm thick confocal section of a propidium iodide–stained protoplast preparation of a tobacco cell line uniformly expressing GFP after stable transformation with MGFPM. Intact protoplasts exclude propidium iodide (PI), whereas free nuclei and nuclei in protoplasts with compromised plasma membrane integrity stain brightly. GFP is absent from protoplasts in which nuclei stain brightly with PI. The arrow indicates a missing portion of the plasma membrane.
(B) Green fluorescence histogram of wild-type protoplasts. Fluorescence was measured in relative fluorescence units (RFU). Because in wild-type cells green fluorescence intensity is below 17 RFU, transformed cells with green fluorescence below 17 RFU are considered GFP negative cells (Neg), and cells with green fluorescence above 17 RFU are considered GFP positive cells (Pos).
(C) MGFPM stably transformed protoplasts stained with PI subjected to biparametric analysis of side light scatter (SSC) versus forward light scatter (FSC). Gated events inside the oval are plotted in (D).
(D) Green fluorescence histogram of gated MGFPM protoplasts from (C).
(E) The same data from the same MGFPM stably transformed protoplasts stained with PI were subjected to biparametric analysis of side light scatter versus relative red fluorescence. Gated events inside the oval are plotted in (F). These gated events have background levels of PI fluorescence similar to that of wild-type cells (data not shown).
(F) Green fluorescence histogram of gated MGFPM protoplasts from (E). Note that after exclusion of protoplasts and debris that have high levels of PI fluorescence, the mode consistent with wild-type fluorescence was lost.