Table 1.
Scope for the synthesis of (un)symmetrical diaryliodonium salts in flow.[a]
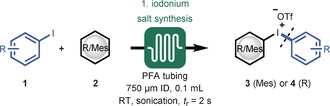
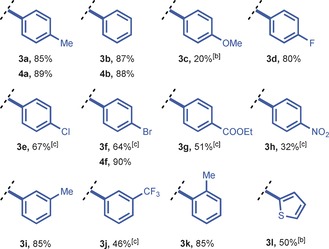
|
[a] Reaction conditions: Syringe 1: 5.0 mmol of aryl iodide (1) and 5.5 mmol of arene (2) in 25 mL DCE at 0.75 mL min−1; syringe 2: 5.5 mmol of m‐CBPA in 25 mL of DCE at 0.75 mL min−1; syringe 3: 10.0 mmol TfOH in 50 mL DCE at 1.5 mL min−1. Added to the reactor by syringe pump. [b] Mesityl iodide was used. [c] 3 mL reactor volume with t r=60 s, 6.5 mmol m‐CBPA, and 15 mmol TfOH. Note: The 3 series refers to unsymmetrical diaryliodonium salts with mesitylene as arene, and the 4 series to symmetrical diaryliodonium salts. m‐CPBA=m‐chloroperbenzoic acid, Tf=trifluoromethanesulfonyl.
