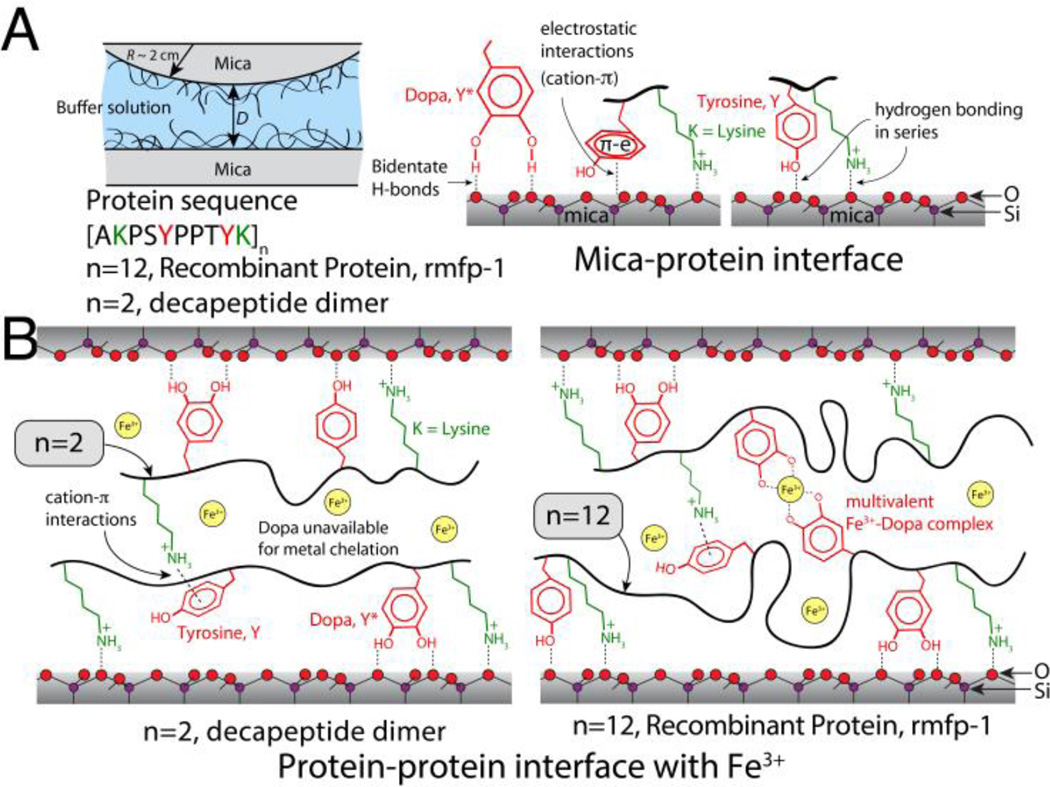
Fig. 1.
Scheme of the surfaces analyzed by the surface forces apparatus. (A) rmfp-1 and short peptides with or without Dopa are adsorbed as thin films onto one or both mica surfaces; Schematics of the bidentate H-bonds, electrostatic and π-cation interactions between the protein and K+ ions adsorbed (not shown for the sake of clarity) to the mica surface (see supplementary Fig. S1). Our results suggest that electrostatic, π-cation and hydrophobic interactions between aromatic residues and mica are more probable than bidentate H-bonding interactions. (B) Schematics showing the effect of peptide length on the adhesive interactions between the protein films. Metal mediated cross-links across the films are possible for proteins containing Dopa residues only when the number of decapeptide monomers is greater than a critical number (n between 2 and 12). For the short decapeptide dimers, most Dopa residues get recruited to the substrate whereas for the decapeptide 12-mer, free Dopa residues remaining at the protein-solution interface are available to bridge with exposed Dopa on the opposing surface through Fe3+ mediated chelation.