Figure 1.
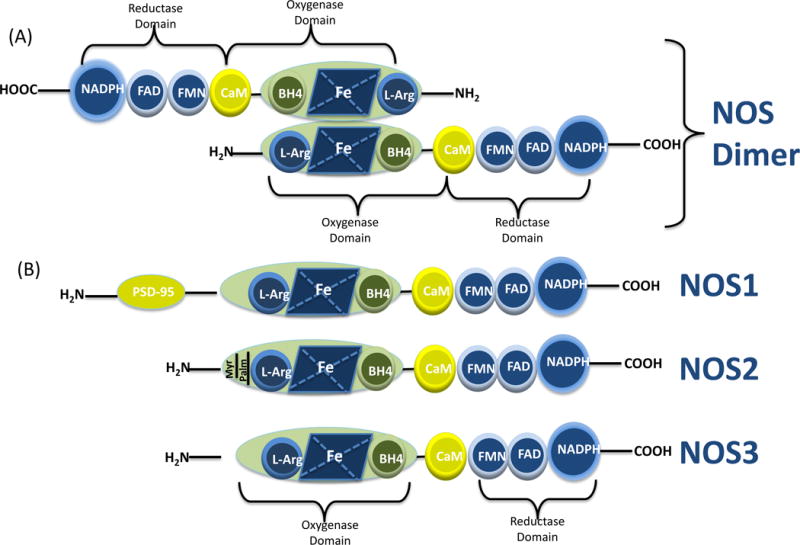
(A) Illustration of the molecular structure of NOS dimer. All NOS monomers include an oxygenase and a reductase domain and calmodulin (CaM) binding site. The oxygenase moiety comprises the L-arginine, heme, and tetrahydrobiopterin (BH4)-binding domains; whereas the reductase moiety includes the flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide phosphate (NADPH)- binding domains. NOS monomers are unable to bind the cofactor BH4 or the substrate arginine to catalyze the NO production. In the presence of heme, NOS can form a functional dimer that can bind to BH4 and L-Arg that allows interdomain electron transfer. (B) Schematic illustration of the protein structure of NOS isoforms.
