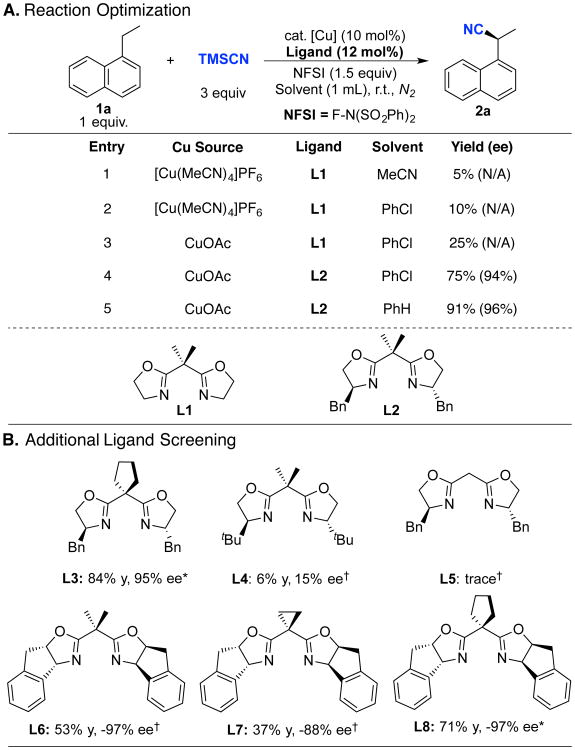
Figure 2. Reaction optimization and representative ligand screening data.
General reaction conditions: 1a (0.2 mmol), TMSCN (0.6 mmol), NFSI (0.3 mmol), CuOAc (0.02 mmol), ligand (0.024 mmol) in 1.0 mL of solvent at r.t. for 10 h. Yield determined by 1H NMR, CF3CONMe2 as internal standard. The ee (enantiomertic excess) value was determined by HPLC with a chiral stationary phase. * Reaction conducted in PhH; † Reaction conducted in PhCl.