Abstract
While household air pollution from biomass fuel combustion has been linked to cardiovascular disease, the effects on cardiac structure and function have not been well described. We sought to determine the association between biomass fuel smoke exposure and cardiac structure and function by transthoracic echocardiography. We identified a random sample of urban and rural residents living in the high-altitude region of Puno, Peru. Daily biomass fuel use was self-reported. Participants underwent transthoracic echocardiography. Multivariable linear regression was used to examine the relationship of biomass fuel use with echocardiographic measures of cardiac structure and function, adjusting for age, sex, height, body mass index, diabetes, physical activity, and tobacco use. One hundred and eighty-seven participants (80 biomass fuel users and 107 non-users) were included in this analysis (mean age 59 years, 58% women). After adjustment, daily exposure to biomass fuel smoke was associated with increased left ventricular internal diastolic diameter (P=.004), left atrial diameter (P=.03), left atrial area (four-chamber) (P=.004) and (two-chamber) (P=.03), septal E′ (P=.006), and lateral E′ (P=.04). Exposure to biomass fuel smoke was also associated with worse global longitudinal strain in the two-chamber view (P=.01). Daily biomass fuel use was associated with increased left ventricular size and decreased left ventricular systolic function by global longitudinal strain.
Keywords: biomass fuel, cardiac function, cardiology, echocardiography, Latin America, Peru
1 INTRODUCTION
Paradoxically, while high-income countries have a greater prevalence of cardiovascular risk factors, low-and middle-income countries have a higher rate of major cardiovascular events, including cardiovascular-related death, myocardial infarction, and stroke.1 Additionally, within middle-income countries, a similar paradox exists between rural and urban dwellers in which rural residents have fewer cardiovascular risk factors but a higher major cardiovascular event rate.1 Particularly in resource-poor settings, identifying elements of the physical environment that increase cardiovascular risk, such as air pollution, provides an opportunity to reduce cardiovascular disease risk at the population level.
Data supporting the association between biomass smoke fuel exposure and cardiovascular disease outcomes are emerging. Household air pollution exposure from biomass fuel use is associated with increased systolic and diastolic blood pressure, hypertension prevalence, pro-inflammatory and pro-thrombotic biomarkers, endothelial dysfunction, heart rate variability, carotid intimal-medial thickness, and ST changes on electrocardiogram.2–19 Fewer studies have examined the relationship between biomass fuel smoke exposure and clinical cardiovascular disease outcomes. Two studies in India and Pakistan found that exposure to biomass fuel smoke was associated with acute coronary syndrome and self-reported cardiovascular disease,20,21 yet large cohort studies in Bangladesh and Iran found no association between household air pollution from biomass fuel use and cardiovascular-related mortality.22,23 Despite the lack of robust evidence that links household air pollution exposure to cardiovascular outcomes, the Global Burden of Disease Study estimated the cardiovascular and cerebrovascular impact of household air pollution exposure and determined that household air pollution is the third greatest risk factor for death worldwide.24 However, the global cardiovascular impact of household air pollution exposure was determined based on the estimated concentration of fine particulate matter (PM2.5) released in biomass fuel combustion and the observed cardiovascular risk associated with PM2.5 exposure from ambient air pollution. The analysis of the Global Burden of Disease Study did not take into consideration the inherent differences in smoke from biomass fuel combustion with respect to pollutants released from fossil fuel combustion.25–27 Therefore, the association between household air pollution and the magnitude of the public health impact on cardiovascular disease outcomes has yet to be determined.
In the absence of large-scale, prospective studies on the effect of household air pollution exposure from biomass fuel use on cardiovascular disease outcomes and mortality, it is possible to detect sub-clinical alterations in cardiac structure and function to determine the ways in which household air pollution exposure could lead to adverse cardiovascular outcomes. Asymptomatic left ventricular systolic and diastolic function increases the risk for incident heart failure in multiple longitudinal cohorts.28,29 A greater description of the physiologic changes associated with household air pollution exposure not only has the potential to further the understanding of the negative cardiovascular impact of household air pollution exposure, but also reveals the mechanisms by which adverse cardiovascular outcomes might occur. The objective of this study was to determine the association between exposure to smoke from daily biomass fuel use and cardiac structure and function measured by transthoracic echocardiography in Puno, Peru.
Practical Implications.
Biomass fuels are used by three billion people worldwide, yet the impact of exposure to biomass fuel smoke on cardiovascular disease outcomes is not well described. The objective of this study was to determine the association between biomass fuel smoke exposure on cardiac structure and function in Puno, Peru. After adjusting for potential confounders, daily biomass fuel smoke exposure was associated with increased left atrial size, increased left ventricular size, and decreased left ventricular systolic function. This difference in cardiac structure and function potentially increases the risk of stroke, heart failure, myocardial infarction, and cardiovascular mortality in biomass fuel users.
2 METHODS
2.1 Study setting
The CRONICAS study is a longitudinal cohort study designed to characterize the prevalence of and risk factors for chronic disease in three geographically distinct settings in Peru.30 This study was conducted in a random sample of CRONICAS participants at the Puno, Peru site, and uses sociodemographic data from the baseline round of data collected in 2011 and echocardiographic data that were collected in 2014. Located near the Bolivian border at 3825 m above sea level, Puno consists of a small, urban provincial capital and several rural villages where biomass fuel is widely used for cooking and heating. Puno primarily consists of indigenous, Andean people with both Quechua-and Aymara-speaking populations.
2.2 Study design
Full-time residents in the area aged ≥35 years who provided informed consent were invited to participate in the study. We identified a sex-and age-stratified (35–44, 45–54, 55–64, and ≥65 years) and location-stratified (urban vs rural) sample, and only one participant per household was enrolled. We used a random number generator to select 200 urban CRONICAS participants and 200 rural CRONICAS participants for a total of 400 participants that were eligible to participate in this sub-study. Because the rural communities are geographically dispersed, for logistical purposes we selected rural participants from two rural communities. This study was approved by the Institutional Review Boards at Universidad Peruana Cayetano Heredia, A.B. PRISMA, Bloomberg School of Public Health at Johns Hopkins University, Duke University School of Medicine, and University of California, San Francisco, CA, USA.
2.3 Study procedures
Participants responded to a face-to-face questionnaire based on the World Health Organization STEP methodology to measure sociodemographic information, cardiopulmonary risk factors, and history of cardiopulmonary symptoms during the baseline round of data collection.30 Questionnaires were conducted by nurse fieldworkers in Spanish, Aymara, and Quechua. At the time of the echocardiography examination, nurse fieldworkers measured weight, height, heart rate, and blood pressure. Systolic and diastolic blood pressures were measured using a digital sphygmomanometer (OMRON HEM-780, Osaka, Japan), using the right arm for all measurements with an appropriately sized cuff.
2.4 Echocardiography
Participants underwent transthoracic echocardiography performed by either a senior sonographer or a senior cardiology fellow using a Sonosite M-Turbo ultrasound machine. The transthoracic echocardiography protocol was created specifically for this study and was adapted from the Duke Cardiac Diagnostic Unit protocol (unpublished) with permission from the Director. Parasternal long-axis, parasternal short-axis, apical four-chamber, apical two-chamber, apical three-chamber, and subcostal views were obtained using 2D imaging, continuous-wave spectral Doppler, pulse-wave spectral Doppler, and M-mode imaging. The images were transferred in Digital Imaging and Communications in Medicine (DICOM) format to the Duke Clinical Research Institute Cardiovascular Imaging Core Laboratory and uploaded to the Digisonics Digiview Cardiovascular Imaging reading platform (Digisonics, Inc, Houston, TX, USA). The following echocardiographic measurements were taken: left ventricular internal diameter in diastole, left ventricular internal diameter in systole, left ventricular mass, left ventricular ejection fraction, left atrial anterior-posterior diameter, left atrial area (four-chamber and two-chamber), E/A ratio, lateral and septal E′ velocity, lateral and septal A′ velocity, lateral and septal S′ velocity, right ventricular width at the base and mid-cavity, right ventricular length, right ventricular systolic pressure (RVSP), tricuspid annular plane systolic excursion (TAPSE), and right ventricular outflow tract time to peak velocity. Left ventricular ejection fraction was calculated using the method of disks (Simpson’s rule), which is calculated using planimetry of the left ventricle in the apical four-chamber and apical two-chamber views in end-systole and end-diastole. Left atrial area was calculated by planimetry of the left atrium in both the apical four-chamber and two-chamber views. RVSP was estimated from the tricuspid regurgitant jet velocity with the modified Bernoulli equation. Right atrial pressure was estimated using the maximum and minimum inferior vena cava (IVC) diameter 1 inch from the hepatic vein in the subcostal view before and after inspiration using the following criteria: 3 mm Hg if the maximum IVC diameter is less than or equal to 2 cm and collapses >50% with inspiration; 8 mm Hg if the maximum IVC diameter is greater than 2 cm and collapses >50% with inspiration or the maximum IVC diameter is less than or equal to 2 cm and does not collapse >50% with inspiration; and 15 mm Hg if the maximum IVC diameter is greater than 2 cm and does not collapse >50% with inspiration. Left ventricular mass was determined based on linear measurements on the 2D parasternal long-axis images using the American Society of Echocardiography Equation.31
For strain measurement, transthoracic echocardiography images were also transferred in DICOM format to Philips Xcelera (Philips Medical Systems, Eindhoven, the Netherlands) on the Duke University Echocardiography Laboratory system and from Xcelera to a vendor-independent software package (2D Cardiac Performance Analysis version 4.5; TomTec Imaging Systems, Unterschleissheim, Germany) at frame rate ≥50/s. Longitudinal strain assessments were performed in the apical four-, three-, and two-chamber views.32 Retrospective two-dimensional speckle-tracking longitudinal strain assessments on 2D images have been validated using TomTec software, even when the original study was not intended for this purpose.33 All analyses were performed by a single experienced operator (FA) blinded to the research participant’s exposure status.
2.5 Definitions
The following variables were determined by self-report in a structured questionnaire: daily biomass fuel use, prior diabetes diagnosis, physical activity, and pack-years of tobacco smoking. Daily biomass fuel use was defined as self-reported daily burning of wood or dung for cooking or heating for more than 6 months at any time during the participant’s lifetime. Although the 24-hour mean concentration of air pollutants was not measured in every participant household in this study, prior studies of this cohort have validated that participants reporting biomass fuel use are exposed to higher concentrations of PM2.5 and carbon monoxide.34 Physical activity was determined based on leisure time and transport time domains of International Physical Activity Questionnaire as recommended for Latin American populations.35,36 Alcohol abuse was measured using the Alcohol Use Disorders Identification Test.37 Education was categorized by schooling years (<6 years, 7–11 years, and 12 or more years). Socioeconomic status was defined as wealth index based on household income, assets, and household facilities as previously described.38
2.6 Biostatistical methods
The primary aim was to compare measures of left and right heart structure and function in participants with and without daily exposure to biomass fuel smoke. Baseline characteristics of daily biomass fuel users and non-users were compared using t-test for continuous variables and chi-squared test for categorical variables. Using histograms, Q-Q plots, and the skewness test, each echocardiographic parameter was evaluated for normality. We log-transformed E/A ratio, left ventricular mass and septal E′ velocity because these parameters were not normally distributed. Single variable and multivariable regression analyses were used to model the association between daily biomass fuel use with each echocardiographic parameter. Multivariable models were adjusted for age, sex, body mass index, height, diabetes, pack-years of smoking, and low physical activity. We did not adjust for blood pressure because hypertension is a potential mediator of air pollution exposure and adverse cardiac remodeling. We used linear regression models for all echocardiographic parameters, with the exception for diastolic dysfunction grade where we used ordinal logistic regression. Sensitivity analyses were conducted in which years of education and assets index were included in the multivariable model. Statistical analyses were conducted in STATA 14 (STATA Corp, College Station, TX, USA).
To assess for reproducibility of echocardiographic measurements, 10% of the sample (19 studies) was randomly selected using the STATA 14 samp command. A second echocardiography reader measured the following variables in the selected studies: left ventricular internal diameter in diastole, interventricular septal diameter, left ventricular ejection fraction, left atrial area (four-chamber), septal E′ velocity, and TAPSE. Intraclass correlation was very strong for left atrial area (0.87) and left ventricular internal diameter in diastole (0.90) and moderately strong for TAPSE (0.63). Intraclass correlation was weaker for left ventricular ejection fraction (0.16), interventricular septal diameter (0.07), and septal E′ velocity (−0.89).
3 RESULTS
3.1 Participant characteristics
A total of 187 participants were included in this study, including 80 daily biomass fuel users and 107 non-users (Table 1). While there were no statistically significant differences in age or sex, daily biomass fuel users had lower body mass index, lower diabetes prevalence, less education, and lower wealth index.
TABLE 1.
Characteristics of daily biomass fuel users and non-users in Puno, Peru
| Participant characteristics | Biomass fuel non-users (N=107) | Daily biomass fuel users (N=80) | P-value |
|---|---|---|---|
| Age (mean) | 58 | 60 | .201 |
| Male, n (%) | 46 (43.0) | 33 (41.3) | .81 |
| Body mass index, kg/m2 (mean) | 27.6 | 25.7 | .001 |
| Heart rate, beats per minute (mean) | 76.2 | 73.6 | .11 |
| Hypertension, n (%) | 9 (8.6) | 5 (7.0) | .71 |
| Diabetes, n (%) | 4 (3.9) | 10 (14.3) | .013 |
| Pack-years of tobacco use (mean) | 0.79 | 0.33 | .37 |
| Hazardous alcohol use, n (%) | 15 (14.3) | 10 (14.1) | .97 |
| Low physical activity, n (%) | 83 (79.8) | 50 (69.4) | .12 |
| Less than primary education, n (%) | 9 (8.6) | 45 (62.5) | <.001 |
| Lowest tertile of wealth index, n (%) | 19 (18.1) | 50 (70.4) | <.001 |
3.2 Left heart structure and function
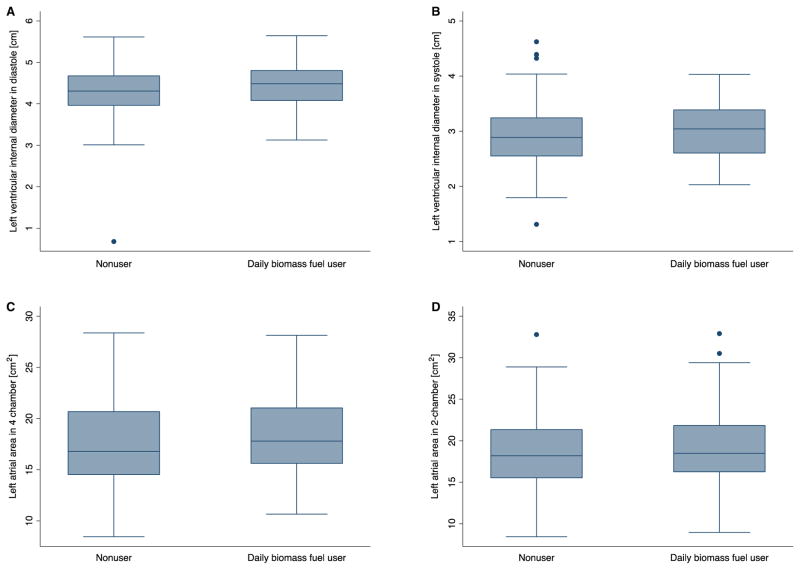
In the unadjusted analysis, none of the echocardiographic measures of left atrial size or left ventricular structure and function were associated with daily biomass fuel use except for the A′ velocity of the septal mitral valve annulus (0.58 m/s: 95% confidence interval [CI] 0.04, 1.12) (Table 2). After adjusting for multiple covariates, however, this association became stronger such that daily biomass fuel use was associated with increased left atrial and left ventricular size. Biomass mass fuel use was associated with a 0.23-cm (95% CI 0.08, 0.38) increase in left ventricular internal diameter at the end of diastole (Figure 1). Left atrial anterior-posterior diameter (0.18 cm: 95% CI 0.02, 0.33) and left atrial area in the apical four-chamber view (1.80 cm2: 95% CI 0.57, 3.03) and apical two-chamber view (1.67 cm2: 95% CI 0.34, 3.01) were also greater in daily biomass fuel users compared with non-users.
TABLE 2.
Unadjusted (single variable) and adjusted (multivariable) regression analyses comparing left atrial and ventricular echocardiographic parameters in daily biomass fuel users and non-users adjusting for age, sex, body mass index, height, physical activity, pack-years of tobacco use, and diabetes in Puno, Peru. We used linear regression models for all echocardiographic parameters, with the exception for diastolic dysfunction grade where we used ordinal logistic regression
| Echocardiographic parameters | Single variable model (95% CI) | P-value | Multivariable model (95% CI) | P-value |
|---|---|---|---|---|
| Left ventricular internal diameter, diastole, cm | 0.10 (−0.06, 0.26) | .20 | 0.23 (0.08, 0.38) | .004 |
| Left ventricular internal diameter, systole, cm | 0.08 (−0.9, 0.25) | .37 | 0.14 (−0.03, 0.32) | .12 |
| Left ventricular end diastolic volume, mL | 1.02 (0.96, 1.08) | .59 | 1.10 (1.05, 1.16) | <.001 |
| Left ventricular end systolic volume, mL | 1.04 (0.96, 1.12) | .34 | 1.13 (1.06, 1.20) | <.001 |
| Left ventricular ejection fraction, % | −0.89 (−2.57, 0.78) | .29 | −0.95 (−2.79, 0.88) | .31 |
| Left ventricular mass, g (exponentiated form) | 0.96 (0.88, 1.04) | .29 | 1.02 (0.95, 1.10) | .57 |
| Left atrial diameter, cm | 0.03 (−0.13, 0.19) | .70 | 0.18 (0.02, 0.33) | .03 |
| Left atrial area, four-chamber, cm2 | 0.58 (−0.67, 1.84) | .36 | 1.80 (0.57, 3.03) | .004 |
| Left atrial area, two-chamber, cm2 | 0.46 (−0.96, 1.89) | .52 | 1.67 (0.34, 3.01) | .01 |
| E/A ratio (exponentiated form) | 1.04 (0.96, 1.13) | .28 | 1.03 (1.02, 1.17) | .01 |
| Lateral E′, cm/s | 0.43 (−0.20, 1.50) | .13 | 0.71 (0.04, 1.38) | .04 |
| Lateral A′, cm/s | −0.26 (−0.92, 0.40) | .44 | −0.20 (−0.87, 0.48) | .57 |
| Lateral S′, cm/s | −0.10 (−0.68, 0.48) | .73 | −0.10 (−0.71, 0.51) | .74 |
| Septal E′, cm/s (exponentiated form) | 1.08 (1.00, 1.18) | .05 | 1.10 (1.03, 1.19) | .006 |
| Septal A′, cm/s | 0.58 (0.04, 1.12) | .04 | 0.37 (−0.17, 0.91) | .18 |
| Sepal S′, cm/s | 0.29 (0.17, 0.74) | .22 | 0.25 (−0.21, 0.70) | .29 |
| Diastolic Dysfunction Grade, Odds ratio | 0.73 (0.40, 1.32) | .30 | 0.41 (0.17, 0.96) | .04 |
| Left atrial strain (two-chamber), % | −0.14 (3.29, −3.57) | .93 | 0.52 (4.28, −3.25) | .79 |
| Left atrial strain (four-chamber), % | 0.54 (4.20, −3.12) | .77 | 0.80 (4.60, 2.99) | .68 |
| Left ventricular strain (global longitudinal), % | 0.47 (−0.17, 1.12) | .15 | 0.47 (−0.25, 1.18) | .20 |
| Left ventricular strain (four-chamber), % | 0.04 (−0.85, 0.93) | .93 | 0.09 (−0.89, 1.07) | .87 |
| Left ventricular strain (three-chamber), % | −0.17 (−1.25, 0.91) | .76 | 0.06 (−1.15, 1.26) | .93 |
| Left ventricular strain (two-chamber), % | 1.36 (0.51, 2.20) | .002 | 1.25 (0.28, 2.22) | .01 |
FIGURE 1.
Left ventricular internal diameter at end-diastole (a), left ventricular diameter at end-systole (b) and left atrial area (four-chamber (c) and two-chamber (d)) in biomass fuel users and non-users
Daily biomass fuel use was associated with changes in left ventricular diastolic function and systolic function. E/A ratio was increased in daily biomass fuel users (1.03: 95% CI 1.02, 1.17). Additionally, both lateral and septal peak E′ velocities were increased in daily biomass fuel users when compared to non-users (0.71 cm/s: 95% CI 0.04, 1.38; 1.10 cm/s: 95% CI 1.03, 1.19, respectively). Yet despite improved diastolic function, daily biomass fuel use was associated with decreased left ventricular systolic function as measured by global longitudinal strain in the apical two-chamber view (1.2%: 95% CI 0.28, 2.22). The difference in global longitudinal strain between biomass fuel users and non-users persisted in sensitivity analyses that incorporated education and assets into the multivariable linear regression analysis (Table S1). However, there was no association between daily biomass fuel use and left ventricular ejection fraction.
3.3 Right heart structure and function
There was no association between daily biomass fuel use and measures of right ventricular size and function, including right ventricular and right atrial strain in either unadjusted or adjusted analyses (Table 3). There was a trend toward increased right ventricular width at the base (0.18 cm: 95% CI −0.01, 0.36) and length (0.25 cm: 95% CI −0.01, 0.51) in daily biomass fuel users in the fully adjusted analysis.
TABLE 3.
Unadjusted (single variable) and adjusted (multivariable) linear regression analyses comparing right atrial and ventricular echocardiographic parameters in daily biomass fuel users and non-users adjusting for age, sex, body mass index, height, physical activity, pack-years of tobacco use, and diabetes in Puno, Peru
| Echocardiographic parameters | Single variable model (95% CI) | P-value | Multivariable model (95% CI) | P-value |
|---|---|---|---|---|
| Right ventricular diameter, base, cm | 0.10 (−0.09, 0.28) | .32 | 0.18 (−0.01, 0.36) | .06 |
| Right ventricular diameter, mid-cavity, cm | −0.02 (−0.19, 0.16) | .86 | 0.11 (−0.06, 0.27) | .20 |
| Right ventricular length, cm | −0.002 (0.27, 0.27) | .99 | 0.25 (−0.01, 0.51) | .06 |
| RVSP, mm Hg | 0.77 (−3.28, 1.74) | .55 | 0.16 (−2.48, 2.80) | .91 |
| TAPSE, cm | −0.04 (−0.14, 0.07) | .52 | 0.05 (−0.06, 0.17) | .37 |
| Right ventricular outflow tract time to peak velocity, ms | 3.50 (−4.97, 11.98) | .42 | 6.25 (−3.20, 15.71) | .19 |
| Right ventricular global longitudinal strain, % | 0.86 (1.82, −0.10) | .08 | 0.62 (1.66, −.042) | .24 |
| Right atrial strain (four-chamber) % | 5.27 (14.68, −4.13) | .27 | 2.57 (13.48, −8.34) | .64 |
RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
4 DISCUSSION
We found an association between self-reported daily biomass fuel use with increased left ventricular and left atrial size by transthoracic echocardiography in a population-based sample of high-altitude dwellers in Peru. Daily biomass fuel use was also associated with decreased left ventricular systolic function as measured by global longitudinal strain. In contrast, we did not find a link between biomass fuel smoke exposure with right heart function or RVSP.
While additional echocardiographic studies in association with household air pollution exposure are ongoing, there are very few published studies that examine the changes in cardiac structure and function that are associated with exposure to smoke from biomass fuel use. Previous studies from our group have examined the relationship of biomass fuel use with RVSP and serum NT-pro-BNP levels in a sample of 153 participants in Puno,39 and found no relationship, which is consistent with the findings of this study. However, a cross-sectional study in Turkey observed increased pulmonary artery systolic pressure and decreased right ventricular and left ventricular function as measured by myocardial performance indices in self-reported chronic biomass fuel users in comparison with non-users. 40 While our study did not measure myocardial performance index, we observed a difference in global longitudinal strain in the two-chamber view, which is a measure left ventricular systolic function of clinical importance. Reduced global longitudinal strain is associated with multiple adverse cardiovascular outcomes including all-cause mortality.41 However, the difference in the RVSP results of the two studies might be due to differing sampling methods. The sample used by Kargin et al. was smaller (N=77) with only female participants and subject to additional selection bias as it was derived from a medical center patient population rather than the general population.
The increase in left atrial and left ventricular size in daily biomass fuel users in comparison with non-users represents a remodeling in cardiac structure that is associated with future adverse cardiovascular outcomes. While often a subclinical finding, left atrial enlargement is can be an early sign of increased left ventricular pressure in diastole, signifying an increase in left ventricular stiffness. Interestingly, in our study diastolic function was better in daily biomass fuel users compared with non-users, suggesting that the increase in left atrial size might reflect a primary insult to the myocardium, rather than a response to increased left ventricular diastolic pressure. Similarly, the observed trend toward increased right ventricular size in the absence of increased RVSP also suggests primary myocardial pathology. Independent of the etiology, left atrial enlargement is associated with increased risk of stroke, myocardial infarction, heart failure, and cardiovascular mortality in multiple population-based cohorts, including the Framingham Heart Study and the Strong Heart Study.42–47 In our study in Peru, the mean left atrial size of daily biomass fuels users remains within the normal range, yet a rightward shift in the population distribution curve of left atrial size could translate into increased risk for cardiovascular events in a greater number of individual daily biomass fuel users compared with non-users.
Increased left ventricular size has been associated in a greater risk for heart failure in two cohorts. Results from the Framingham Heart Study and the Framingham Offspring Study showed that left ventricular end diastolic dimension was associated with an increased risk of heart failure in individuals with no history of prior myocardial infarction.48 Similarly, in the Multi-Ethnic Study of Atherosclerosis left ventricular dilatation measured by cardiac magnetic resonance imaging was associated with increased incident heart failure independent of comorbidities and left ventricular ejection fraction.49 These data suggest that an increase in the mean left ventricular size in biomass fuel users compared with non-users might increase the risk for heart failure even in the presence of normal left ventricular systolic function. However, longitudinal follow-up of our cohort is necessary to determine whether the observed increases in left atrial and left ventricular size predict adverse cardiovascular outcomes in this population.
The changes in cardiac structure and function in association with biomass fuel use has implications for both public health policy and energy policy. Due to the widespread use of biomass fuels for cooking and heating in low-income communities throughout the developing world, there has been increased interest in clean cookstove interventions on a global scale. Yet, despite large-scale investment to improve cookstoves and cultural adaptation, the totality of the health impact of household air pollution from biomass fuels use remains unknown. This uncertainty is important to highlight, because there are no clear guidelines for what constitutes a successful cookstove intervention, in terms of both reduction in the exposure to airborne pollutants and the anticipated health impact of any given reduction in the exposure. Better understanding of the way in which household air pollution increases risk for cardiovascular disease provides greater justification for investing in interventions to reduce biomass fuel smoke exposure, and also provides benchmarks with which we can evaluate the efficacy of clean cook-stove interventions to improve health outcomes in low-income communities. Reducing exposure to household air pollution as a strategy for cardiovascular disease prevention has been largely omitted from the global non-communicable disease agenda.50,51 Rigorous studies of cardiovascular risk factors that disproportionately affect low-and middle-income countries, such as household air pollution, and research into the best methods for reducing the associated cardiovascular morbidity and mortality have been advocated but largely not carried out.52
This study is an observational study and thus cannot determine causation. Daily biomass fuel users in Puno are more likely than non-users to live in rural communities, and therefore, unmeasured confounders potentially exist.16,34 Moreover, while both groups in our sample lived at similar altitudes, it remains unclear whether extreme altitude affects the relationship between biomass fuel use and cardiac structure and function. Studies of Sherpas living in high-altitude regions of Nepal found that compared with Sherpas living at lower altitudes, higher-altitude dwelling Sherpas had decreased left ventricular size and decreased diastolic function, but preserved systolic function.53 However, Sherpa populations at both altitudes had worse diastolic function compared with Caucasian populations living at low altitudes, suggesting inherited traits related to cardiac structure and function that are specific to the Sherpa population. 54 While chronic exposure to high altitude might exert an effect on baseline cardiac structure and function in the Andean population in this study, the observed changes in cardiac structure and function associated with biomass fuel smoke exposure appear to counter to the effect of high altitude. This study is also limited by a modest sample size and potentially subject to selection bias in that the rural participants were only selected from two rural communities. Additionally, we used self-reported daily biomass fuel use as proxy for household air pollution exposure and did not measure the individual particulate matter and gaseous components of biomass fuel combustion smoke to determine a dose-response and identify which pollutant is more greatly associated with changes in cardiac structure and function. However, prior studies conducted in Puno have demonstrated that mean household PM2.5 and CO levels are substantially greater daily biomass fuel users in comparison with non-users in the CRONICAS Cohort Study, and the mean concentration of PM2.5 in urban Puno is relatively low at 23 μg/m3.34 Any misclassification of the exposure biases the results toward the null; therefore, we are likely underestimating the true effect of exposure to biomass smoke on cardiac structure and function. Despite the limitations, this study is the first study to examine cardiac structure and function in relation to biomass fuel smoke exposure in a population-based cohort. Additionally, the echocardiographic images were acquired systematically in a robust manner by highly trained personnel from a high-volume academic echocardiography laboratory.
5 CONCLUSIONS
Daily biomass fuel use was associated with increased left ventricular, reduced left ventricular global longitudinal strain, and increased left atrial size with no associated change in right heart size and function in individuals living at high altitude in Peru. These changes in cardiac structure have the potential to increase risk of multiple cardiovascular outcomes, including stroke, heart failure, myocardial infarction, and cardiovascular mortality in biomass fuel users at the population level, with implications for the wider global health and energy policy agendas. Better understanding of the relationship between biomass fuel use, cardiac remodeling, and cardiovascular outcomes could improve the assessment of clean cookstove interventions as a population cardiovascular disease prevention strategy.
Supplementary Material
Acknowledgments
Funding information
This project has been funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900033C. Melissa Burroughs Peña was supported by NIH Research Training Grant R25TW009337 funded by the Fogarty International Center and the National Institute of Mental Health in addition to the Vanderbilt-Emory-Cornell-Duke Consortium, the Fogarty International Center, and the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
CONFLICT OF INTERESTS
The authors disclose no conflict of interests.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 2.Barregard L, Sallsten G, Gustafson P, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–853. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- 3.Ray MR, Mukherjee S, Roychoudhury S, et al. Platelet activation, upregulation of CD11b/CD18 expression on leukocytes and increase in circulating leukocyte-platelet aggregates in Indian women chronically exposed to biomass smoke. Hum Exp Toxicol. 2006;25:627–635. doi: 10.1177/0960327106074603. [DOI] [PubMed] [Google Scholar]
- 4.McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davutoglu V, Zengin S, Sari I, et al. Chronic carbon monoxide exposure is associated with the increases in carotid intima-media thickness and C-reactive protein level. Tohoku J Exp Med. 2009;219:201–206. doi: 10.1620/tjem.219.201. [DOI] [PubMed] [Google Scholar]
- 6.Allen RW, Carlsten C, Karlen B, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–1230. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 7.Dutta A, Mukherjee B, Das D, Banerjee A, Ray MR. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air. 2011;21:165–176. doi: 10.1111/j.1600-0668.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 8.McCracken J, Smith KR, Stone P, Diaz A, Arana B, Schwartz J. Intervention to lower household wood smoke exposure in Guatemala reduces ST-segment depression on electrocardiograms. Environ Health Perspect. 2011;119:1562–1568. doi: 10.1289/ehp.1002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee A, Mondal NK, Das D, Ray MR. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation. 2012;35:671–683. doi: 10.1007/s10753-011-9360-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, Hang JQ, Zhang FY, et al. Household solid fuel use and pulmonary function in an urban population in Shanghai, China. Occup Environ Med. 2013;70:120–125. doi: 10.1136/oemed-2011-100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Painschab MS, Davila-Roman VG, Gilman RH, et al. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart. 2013;99:984–991. doi: 10.1136/heartjnl-2012-303440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YL, Chen HW, Han BC, et al. Personal exposure to household particulate matter, household activities and heart rate variability among housewives. PLoS One. 2014;9:e89969. doi: 10.1371/journal.pone.0089969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander D, Larson T, Bolton S, Vedal S. Systolic blood pressure changes in indigenous Bolivian women associated with an improved cookstove intervention. Air Qual Atmos Health. 2015;8:47–53. [Google Scholar]
- 16.Burroughs Pena M, Romero KM, Velazquez EJ, et al. Relationship between daily exposure to biomass fuel smoke and blood pressure in high-altitude Peru. Hypertension. 2015;65:1134–1140. doi: 10.1161/HYPERTENSIONAHA.114.04840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caravedo MA, Herrera PM, Mongilardi N, et al. Chronic exposure to biomass fuel smoke and markers of endothelial inflammation. Indoor Air. 2016;26:768–775. doi: 10.1111/ina.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris C, Goldberg MS, Marshall JD, et al. A panel study of the acute effects of personal exposure to household air pollution on ambulatory blood pressure in rural Indian women. Environ Res. 2016;147:331–342. doi: 10.1016/j.envres.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Quinn AK, Ae-Ngibise KA, Jack DW, et al. Association of Carbon Monoxide exposure with blood pressure among pregnant women in rural Ghana: evidence from GRAPHS. Int J Hyg Environ Health. 2016;219:176–183. doi: 10.1016/j.ijheh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firdaus G, Ahmad A. Indoor air pollution and self-reported diseases— a case study of NCT of Delhi. Indoor Air. 2011;21:410–416. doi: 10.1111/j.1600-0668.2011.00715.x. [DOI] [PubMed] [Google Scholar]
- 21.Fatmi Z, Coggon D, Kazi A, Naeem I, Kadir MM, Sathiakumar N. Solid fuel use is a major risk factor for acute coronary syndromes among rural women: a matched case control study. Public Health. 2014;128:77–82. doi: 10.1016/j.puhe.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam D, Chowdhury M, Siddiquee A, et al. Adult cardiopulmonary mortality and indoor air pollution: a 10-year retrospective cohort study in a low-income rural setting Global. Heart. 2012;7:215–221. doi: 10.1016/j.gheart.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Mitter S, Vedanthan R, Islami F, et al. Household fuel use and cardiovascular disease mortality: Golestan Cohort Study. Circulation. 2016;133:2360–2369. doi: 10.1161/CIRCULATIONAHA.115.020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng Q, Richmond-Bryant J, Lu SE, et al. Cardiovascular outcomes and the physical and chemical properties of metal ions found in particulate matter air pollution: a QICAR study. Environ Health Perspect. 2013;121:558–564. doi: 10.1289/ehp.1205793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnett RT, Pope CA, 3rd, Ezzati M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122:397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KR, Bruce N, Balakrishnan K, et al. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 28.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta-analysis. JACC Heart Fail. 2016;4:237–248. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Tsao CW, Lyass A, Larson MG, et al. Prognosis of adults with borderline left ventricular ejection fraction. JACC Heart Fail. 2016;4:502–510. doi: 10.1016/j.jchf.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W, Group CCS. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Risum N, Ali S, Olsen NT, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–1203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Pollard SL, Williams DL, Breysse PN, et al. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health. 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallal PC, Gomez LF, Parra DC, et al. Lessons learned after 10 years of IPAQ use in Brazil and Colombia. J Phys Act Health. 2010;7(Suppl 2):S259–S264. doi: 10.1123/jpah.7.s2.s259. [DOI] [PubMed] [Google Scholar]
- 36.Miranda JJ, Carrillo-Larco RM, Gilman RH, et al. Patterns and determinants of physical inactivity in rural and urban areas in Peru: a population-based study. Journal of Physical Activity & Health. 2016;13:654–662. doi: 10.1123/jpah.2015-0424. [DOI] [PubMed] [Google Scholar]
- 37.Gomez Arnaiz A, Conde Martela A, Alberto Aguiar Bautista J, Manuel Santana Montesdeoca J, Jorrin Moreno A, Betancor Leon P. Diagnostic usefulness of Alcohol Use Disorders Identification Test (AUDIT) for detecting hazardous alcohol consumption in primary care settings. Med Clin. 2001;116:121–124. doi: 10.1016/s0025-7753(01)71745-9. [DOI] [PubMed] [Google Scholar]
- 38.Howe LD, Galobardes B, Matijasevich A, et al. Measuring socio-economic position for epidemiological studies in low-and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–886. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caravedo MA, Painschab MS, Davila-Roman VG, et al. Lack of association between chronic exposure to biomass fuel smoke and markers of right ventricular pressure overload at high altitude. Am Heart J. 2014;168:731–738. doi: 10.1016/j.ahj.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kargin R, Kargin F, Mutlu H, et al. Long-term exposure to biomass fuel and its relation to systolic and diastolic biventricular performance in addition to obstructive and restrictive lung diseases. Echocardiography. 2011;28:52–61. doi: 10.1111/j.1540-8175.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 41.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 43.Tsang TS, Barnes ME, Gersh BJ, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 44.Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Bangalore S, Yao SS, Chaudhry FA. Role of left atrial size in risk stratification and prognosis of patients undergoing stress echocardiography. J Am Coll Cardiol. 2007;50:1254–1262. doi: 10.1016/j.jacc.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102:70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bombelli M, Facchetti R, Cuspidi C, et al. Prognostic significance of left atrial enlargement in a general population: results of the PAMELA study. Hypertension. 2014;64:1205–1211. doi: 10.1161/HYPERTENSIONAHA.114.03975. [DOI] [PubMed] [Google Scholar]
- 48.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 49.Yeboah J, Bluemke DA, Hundley WG, Rodriguez CJ, Lima JA, Herrington DM. Left ventricular dilation and incident congestive heart failure in asymptomatic adults without cardiovascular disease: multi-ethnic study of atherosclerosis (MESA) J Cardiac Fail. 2014;20:905–911. doi: 10.1016/j.cardfail.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce N, Ebrahim S, McKee M, et al. The road to 25×25: how can the five-target strategy reach its goal? Lancet Glob Health. 2014;2:e126–e128. doi: 10.1016/S2214-109X(14)70015-4. [DOI] [PubMed] [Google Scholar]
- 51.Pearce N, Ebrahim S, McKee M, et al. Global prevention and control of NCDs: limitations of the standard approach. J Public Health Policy. 2015;36:408–425. doi: 10.1057/jphp.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebrahim S, Pearce N, Smeeth L, Casas JP, Jaffar S, Piot P. Tackling non-communicable diseases in low-and middle-income countries: is the evidence from high-income countries all we need? PLoS Med. 2013;10:e1001377. doi: 10.1371/journal.pmed.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stembridge M, Ainslie PN, Shave R. Short-term adaptation and chronic cardiac remodelling to high altitude in lowlander natives and Himalayan Sherpa. Exp Physiol. 2015;100:1242–1246. doi: 10.1113/expphysiol.2014.082503. [DOI] [PubMed] [Google Scholar]
- 54.Stembridge M, Ainslie PN, Donnelly J, et al. Cardiac structure and function in adolescent Sherpa; effect of habitual altitude and developmental stage. Am J Physiol Heart Circ Physiol. 2016;310:H740–H746. doi: 10.1152/ajpheart.00938.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.