Fig. 3.
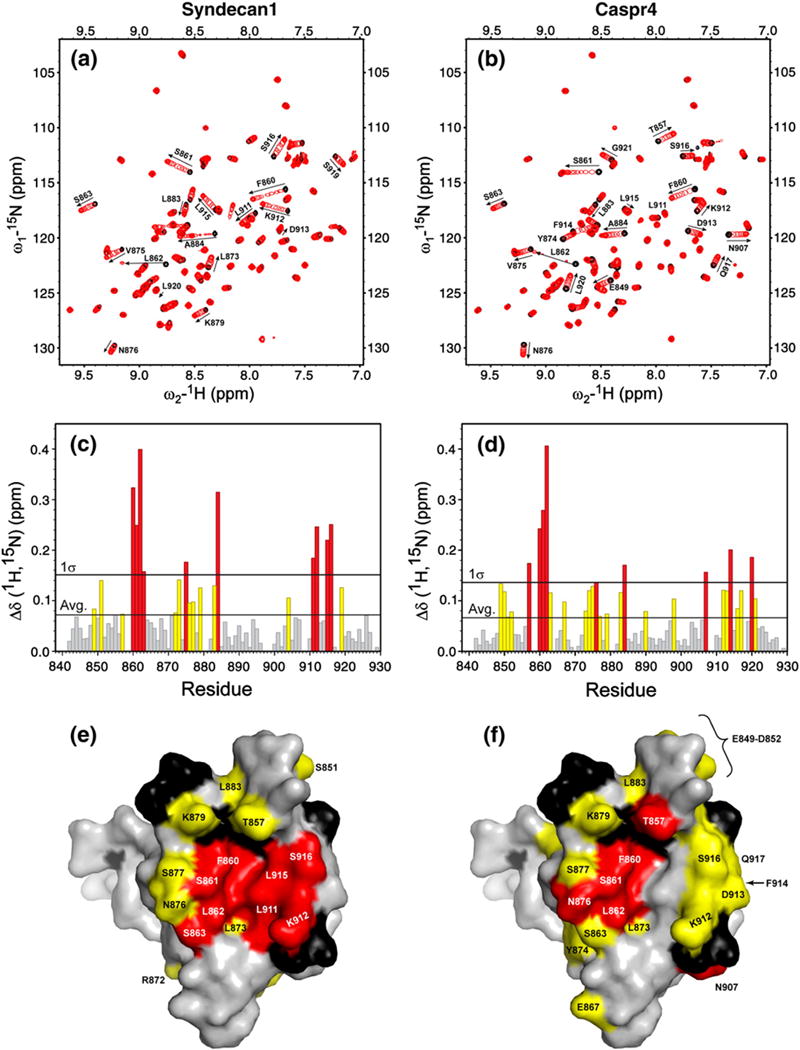
Syndecan1 and Caspr4 peptides induce distinct changes in 1H-15N heteronuclear single quantum coherence spectra of the Tiam1 PDZ domain. The ligand-free protein (black peaks) was titrated with either (a) the Syndecan1 or (b) the Caspr4 peptide (red peaks) to a final PDZ to peptide molar ratio of 1:3. (c and d) The weighted changes in chemical shift upon titration; residues with a chemical shift change >σ above the average are colored red, and residues with a chemical shift change between the average and 1σ are colored yellow. Residues that did not have a significant change in chemical shift are colored gray. The changes in chemical shift for (e) Syndecan1 and (f) Caspr4 are mapped onto the spacefilling model of the Tiam1 PDZ/Model crystal structure. Residues colored red and yellow follow the convention described above. Unassigned residues are black and residues unchanged during the titration are colored gray.
