Fig. 6.
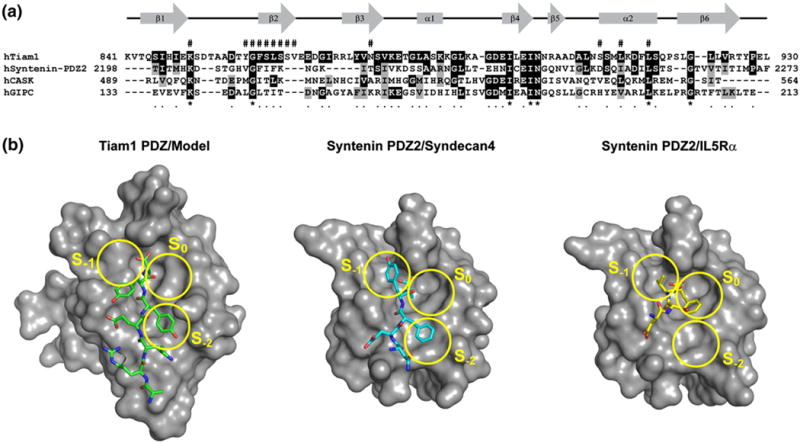
Distinct modes of peptide binding for Tiam1 and Syntenin PDZ domains. (a) The amino acid alignment of Syndecan-binding PDZ domains. Black and gray shading indicate that the residue is identical with or similar to that found in the Tiam1 PDZ domain, respectively. The pound sign (#) above the amino acid sequence indicates residues that participate in Tiam1 PDZ/ligand interactions. The gene accession codes are: Tiam1 (Q13009), Syntenin (O00560), Ca2+/calmodulin-associated Ser/Thr kinase (O14936), and GIPC (014908). (b) The binding surface and ligand-binding modes for the Tiam1 PDZ/Model and Syntenin PDZ2/Syndecan4 (PDB code 1OBY) complexes are compared. The three PDZ binding pockets (S0, S−1 and S−2) are highlighted in yellow and the orientation of each peptide is shown in stick representation. The Syntenin PDZ2/IL5Rα complex (PDB code 1OBX) illustrates how the C-terminal Phe residue of the peptide fits into the S0 pocket.
