Abstract
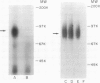
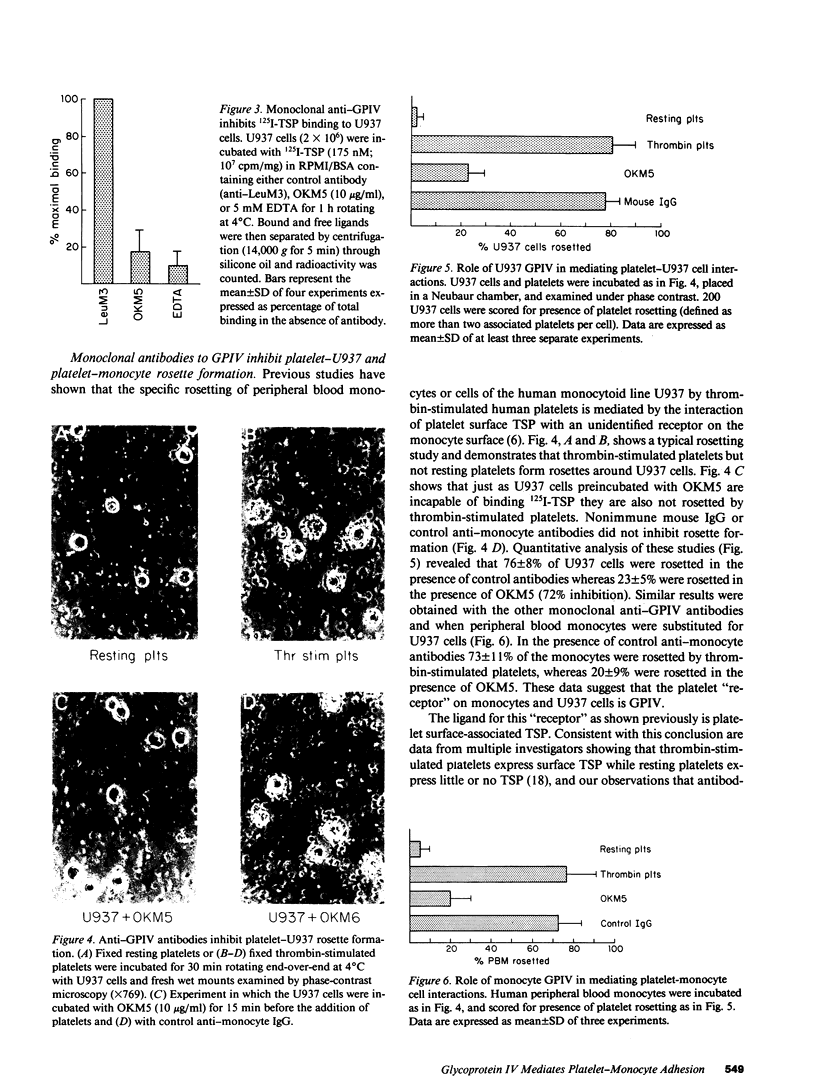
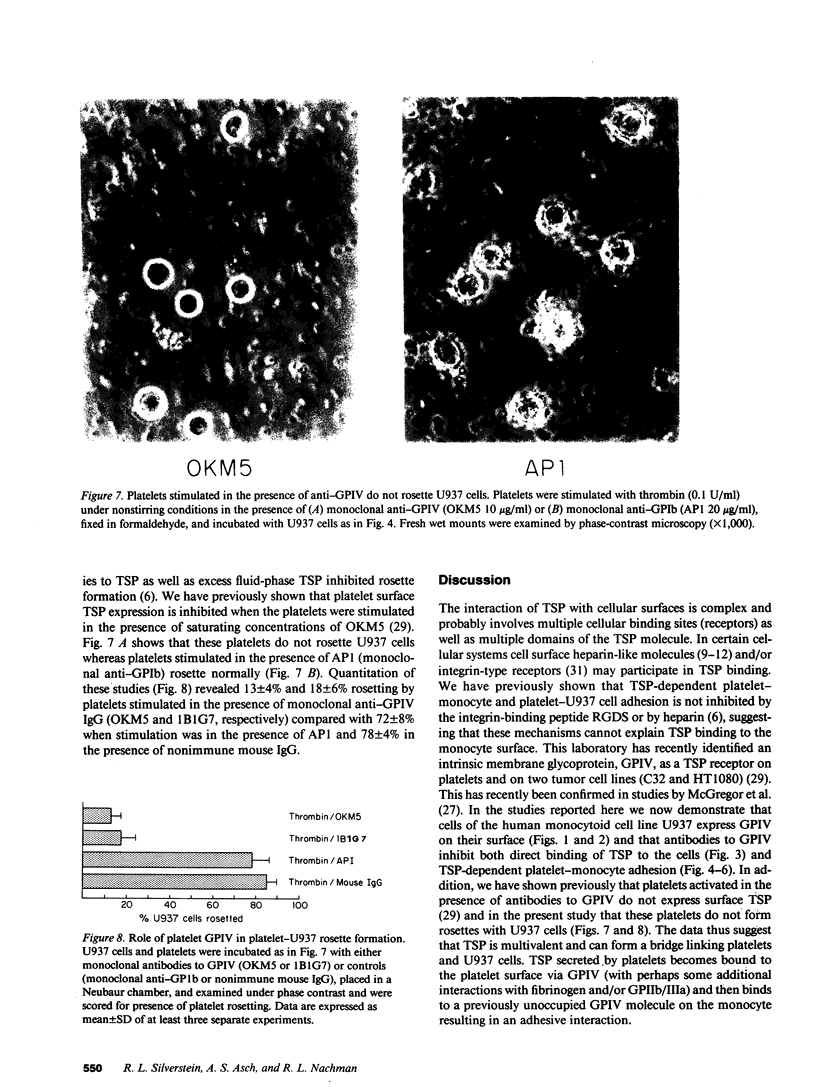
An adhesive interaction between activated platelets and mononuclear phagocytes may contribute to the role these cells play in regulating inflammation, thrombosis, and atherosclerosis. We have previously shown that this adhesive interaction is mediated by the expression of the glycoprotein thrombospondin (TSP) on the surface of activated platelets. We now show that TSP-dependent platelet-monocyte interactions are mediated by glycoprotein IV (GPIV), an intrinsic membrane protein recently identified as a cell surface TSP receptor. Monoclonal antibodies to GPIV bound to cells of the human monocytoid line U937 as assessed by flow cytometry and inhibited the binding of 125I-TSP to the cell surface by 83%. U937 cells preincubated with anti-GPIV were not rosetted by thrombin-stimulated platelets (72% inhibition compared with control anti-monocyte antibodies). In addition, when platelets were stimulated in the presence of saturating concentrations of monoclonal antibodies to GPIV, only 18% of U937 cells were rosetted (78% inhibition). Control antibodies including anti-GPIb did not inhibit rosette formation. These data suggest that TSP can cross-link platelets and monocytes via an interaction with GPIV on the surface of both cells. This molecular bridge may mediate platelet-macrophage communication in various pathophysiologic settings.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Mannucci P. M. Thromboxane generation by human monocytes enhances platelet function. J Exp Med. 1986 Nov 1;164(5):1815–1820. doi: 10.1084/jem.164.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. A monoclonal antibody against human thrombospondin inhibits platelet aggregation. Proc Natl Acad Sci U S A. 1985 May;82(10):3472–3476. doi: 10.1073/pnas.82.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Walz D. A., Aiken M., Starr-Spires L., Ogilvie M. L. Antibodies against a 23Kd heparin binding fragment of thrombospondin inhibit platelet aggregation. Biochem Biophys Res Commun. 1984 Oct 15;124(1):290–295. doi: 10.1016/0006-291x(84)90950-1. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Spycher M. O., Nydegger U. E., Barandun S. Platelet-leukocyte interaction: selective binding of thrombin-stimulated platelets to human monocytes, polymorphonuclear leukocytes, and related cell lines. Blood. 1986 Mar;67(3):629–636. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982 Sep;70(3):542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L., Harpel P. C. Complex formation of platelet thrombospondin with histidine-rich glycoprotein. J Clin Invest. 1984 Jan;73(1):5–12. doi: 10.1172/JCI111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984 Nov;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzet R., Niemetz J., Marcus A. J., Broekman M. J. Enhancement of mononuclear procoagulant activity by platelet 12-hydroxyeicosatetraenoic acid. J Clin Invest. 1986 Aug;78(2):418–423. doi: 10.1172/JCI112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor J. L., Catimel B., Parmentier S., Clezardin P., Dechavanne M., Leung L. L. Rapid purification and partial characterization of human platelet glycoprotein IIIb. Interaction with thrombospondin and its role in platelet aggregation. J Biol Chem. 1989 Jan 5;264(1):501–506. [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Capitanio A., Greenland T., Lüscher E. F., Dechavanne M. Glycoproteins of platelet membranes from Glanzmann's thrombasthenia. A comparison with normal using carbohydrate-specific or protein-specific labelling techniques and high-resolution two-dimensional gel electrophoresis. Eur J Biochem. 1981 May 15;116(2):379–388. doi: 10.1111/j.1432-1033.1981.tb05346.x. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Hanning R., Mosher D. F. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol. 1984 Jan;98(1):22–28. doi: 10.1083/jcb.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987 Oct;105(4):1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Roberts D. D., Haverstick D. M., Dixit V. M., Frazier W. A., Santoro S. A., Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem. 1985 Aug 5;260(16):9405–9411. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984 Nov;74(5):1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Platelet thrombospondin forms a trimolecular complex with plasminogen and histidine-rich glycoprotein. J Clin Invest. 1985 Jun;75(6):2065–2073. doi: 10.1172/JCI111926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Nachman R. L. Thrombospondin: a versatile multifunctional glycoprotein. Arteriosclerosis. 1986 May-Jun;6(3):245–253. doi: 10.1161/01.atv.6.3.245. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L., Leung L. L., Harpel P. C. Activation of immobilized plasminogen by tissue activator. Multimolecular complex formation. J Biol Chem. 1985 Aug 25;260(18):10346–10352. [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V., Murphy A., Siegler K., Smith L., Smith S., Karczewski J., Knudsen K. A. Thrombospondin promotes cell-substratum adhesion. Science. 1987 Jun 19;236(4808):1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Wolff R., Plow E. F., Ginsberg M. H. Interaction of thrombospondin with resting and stimulated human platelets. J Biol Chem. 1986 May 25;261(15):6840–6846. [PubMed] [Google Scholar]