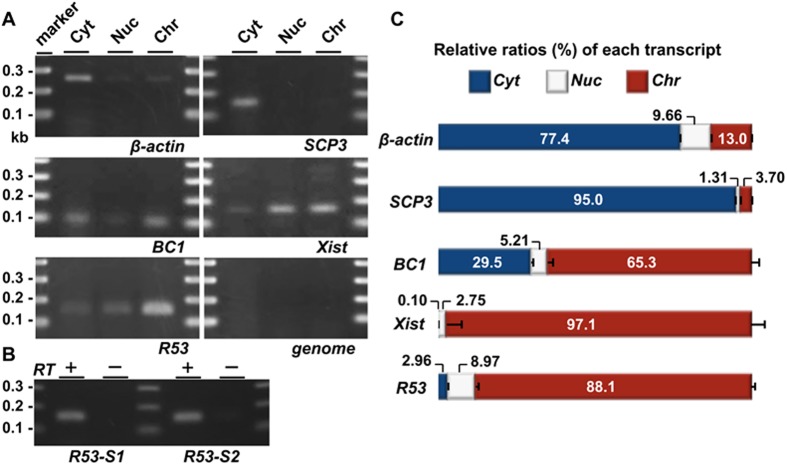
Fig 3. QRT-PCR analyses of the subcellular localization of the RNAs in the testicular cells.
Testicular cells were prepared from the testes of mice 22 days after birth; this stage approximately corresponds to the time of the highest level of R53 expression (Fig 5B). (A) Gel electrophoreses of PCR products using primer pairs for SCP3 (19 cycles), BC1 (22 cycles), Xist (35 cycles), β-actin (18 cycles), R53 RNA (35 cycles, R53-S2 for the primer detecting a R53-B1F-containing sequence downstream of that of R53-S1) and genotyping of the Jmjd1C locus (genome, 35 cycles) in the cytoplasmic extract (Cyt), nuclear soluble (Nuc) and chromatin-bound (Chr) fractions are shown. The genotyping PCR (qJ1C used as the primer) to detect a sequence in the third intron of wild-type Jmjd1C gene was performed to demonstrate that there was almost no contamination of the genomic DNA in any fraction. All the primer pairs used are listed in S1 Table. (B) Reverse-transcription was performed using total RNA prepared from the chromatin-bound fraction and oligo-dT primer with (RT+) or without reverse transcriptase (RT-). As in (A), PCR was performed using 2 sets of primers for R53 RNA (35 cycles) to testify that the PCR products were derived from RT-dependent cDNA. The PCR specificity for R53 transcript was confirmed by sequencing analyses of the PCR products. (C) The amounts of each transcript in the three subcellular fractions were quantitatively analyzed. The values indicated are the relative levels of each transcript against total values of the three subcellular fractions (set as 100%). The error bars indicate the SEM (n = 6).