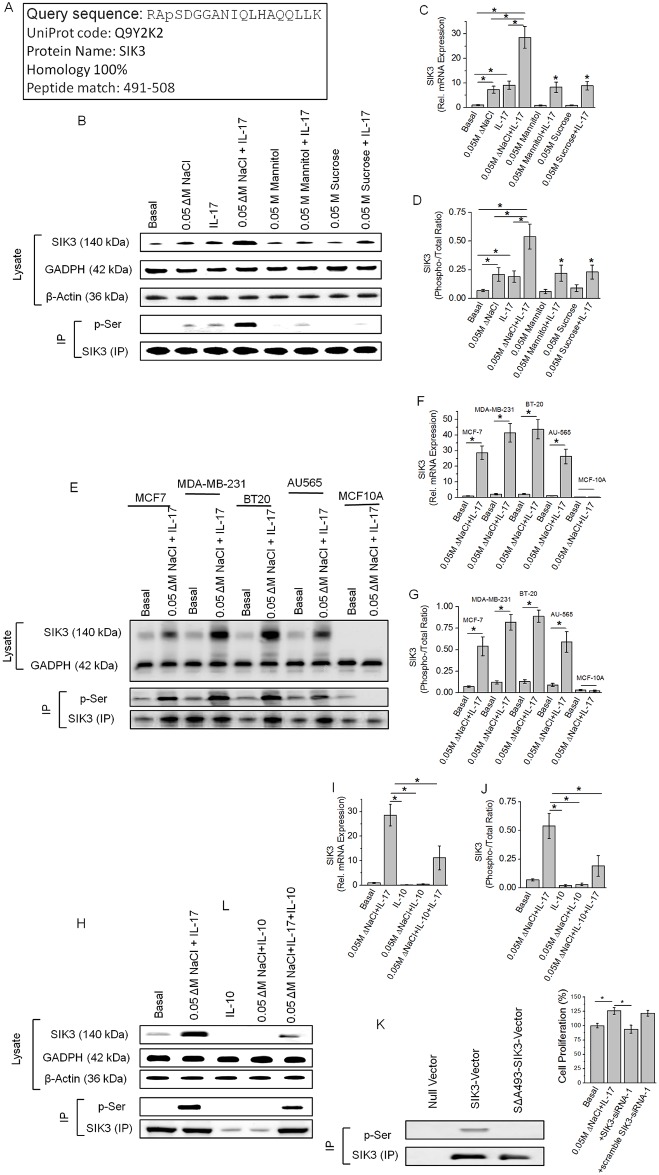
Fig 1. Identification of upregulation of SIK3 specifically following a high salt synergized IL-17 stimulation of breast cancer cells.
(A) Identification of the 1.9 fold higher enriched phospho-peptide sequence following high salt induced stimulation of MCF-7 cells. The identified sequence demonstrated 100% homology to the SIK3 protein at the serine-493 residue. (B) Western blot analysis of SIK3 expression in total cell lysate. Immunoprecipitation of SIK3 is probed with phospho-serine antibody. As can be noted, SIK3 expression was upregulated following treatment with high salt (0.05 M NaCl) and IL-17 (0.1 ng/mL) individually, further, SIK3 expression was synergistically elevated following co-treatment with high salt and IL-17. Equimolar mannitol (0.05 M) and sucrose (0.05 M) were used as negative controls. (C) mRNA transcript expression of SIK3 by ΔΔcT method of quantitative real time polymerase chain reaction normalized for GADPH demonstrated a significant upregulation following high salt (7.3±1.4 fold) and IL-17 (9.1±1.7 fold), and co-treatment with both high salt and IL-17 induced a (28.6±4.4 fold) synergistically enhanced expression. (D) Densitometry quantitation of phospho-SIK3 in (B) demonstrated a synergistically enhanced phosphorylation of SIK3 by high salt and IL-17. (E-G) Expression of SIK3 following co-treatment with high salt and IL-17 in five breast tissue related cell lines (E) were used in our studies, of these, four breast cancer cells (MCF7, MDA-MB-231, BT20, AU565) and one non-malignant breast epithelial cell line (MCF10A); mRNA transcript analysis in 5 cell lines (F); densitometry quantitation of phosphorylated SIK3 in five cell lines (G). (H-J) Anti-inflammatory cytokine interleukin-10 (IL-10) inhibited the expression of SIK3 protein (H), mRNA transcript (I) and phosphorylation of SIK3(J). (K) To demonstrate the phosphorylation is specifically on Serine-493 of SIk3 we clone the SIK3 into MCF10A cell line and latter stimulated by co-treatment with high salt and IL-17. Of note, wild type MCF10A did not demonstrate SIK3 expression following co-treatment with high salt and IL-17 (E-G). Mutation of serine to alanine (SΔA493-SIK3) did not demonstration any binding with phospho-serine in SIK3 immunoprecipitate. (L) Cell proliferation of MCF-7 cells was inhibited following SIK3 siRNA treatment following co-treatment with high salt and IL-17. Of note, we have previously demonstrated that (Ref [6, 13]) co-treatment with high salt and IL-17 induced a 25% higher proliferation of MCF-7 cancer cells. The other three cancer cells MDA-MB-231 (29% higher), BT-20 (31% higher) and AU565 (24% higher) cell proliferation following co-treatment with high salt and IL-17, while non-malignant breast epithelial MCF10A cells did not demonstrate any enhanced proliferation following similar treatment conditions. All data represented as mean values ± SEM from four independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).