Figure 1.
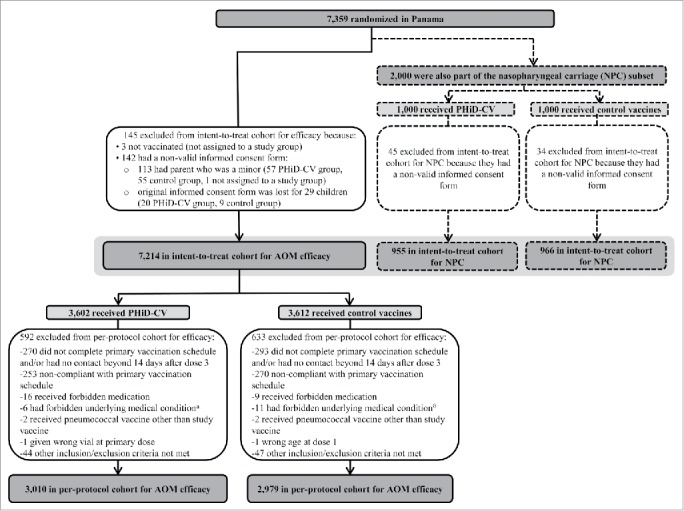
Trial profile for children included in the analysis of acute otitis media (AOM) and nasopharyngeal carriage (NPC). Footnote: Elimination criteria shown for one reason only although more than one reason for elimination could apply per child. For children part of the carriage subset, both efficacy against AOM and impact on nasopharyngeal carriage were assessed. Note that overall, 142 children were excluded from the intent-to-treat cohort due to non-valid informed consent forms, among them; the 79 children excluded also from the NPC intent-to-treat cohort. a Forbidden underlying medical conditions included, but were not limited to: major congenital defects or serious chronic illness, and confirmed or suspected immunosuppressive or immunodeficient condition. AOM, acute otitis media; NPC, nasopharyngeal carriage.
