Figure 3.
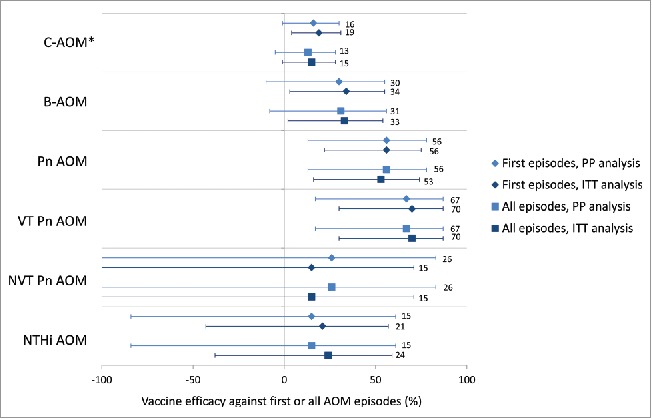
Vaccine efficacy of PHiD-CV against first or all AOM episodes in the per-protocol and intent-to-treat analyses. Footnote: PP: N = 3010 (PHiD-CV); N = 2979 (Control); ITT: N = 3602 (PHiD-CV); N = 3612 (Control). Error bars depict 95% confidence interval; N, number of children in PP or ITT cohort; PP, per-protocol; ITT, intent-to-treat; AOM, acute otitis media; C-AOM, clinically confirmed AOM; B-AOM, bacteriologically confirmed AOM; Pn, S. pneumoniae; VT, vaccine type; NVT, non-vaccine non-vaccine-related type; NTHi, non typeable H. influenzae. *Vaccine efficacy against first C-AOM in per-protocol analysis was assessed as secondary confirmatory objective.
