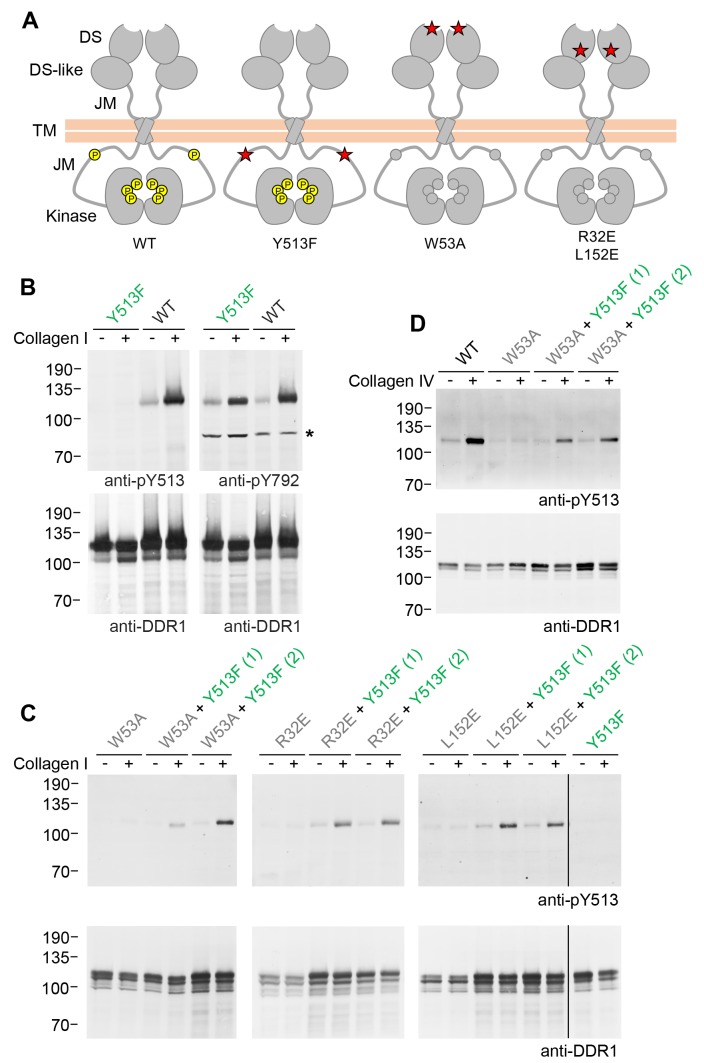
Figure 1. Co-expression of DDR1 donor kinase with signalling-incompetent receiver DDR1 mutants leads to collagen-induced phosphorylation of receiver DDR1.
(A) Schematic diagrams of wild-type and mutant DDR1b. The extracellular region consists of the ligand-binding discoidin (DS) domain, the discoidin-like (DS-like) domain and a flexible juxtamembrane (JM) region. The transmembrane (TM) domain contains a dimerising motif. The cytoplasmic region contains a large unstructured JM region, followed by the catalytic kinase domain. Collagen binding induces phosphorylation of DDR1b on cytoplasmic tyrosine residues; Y513 in the JM region and the three activation loop tyrosines are shown in phosphorylated form for WT DDR1b as yellow circles. DDR1b-Y513F is phosphorylated on the activation loop but cannot be phosphorylated on Y513. Ligand-binding defective DDR1b-W53A and signalling-defective DDR1b-R32E or DDR1b-L152E are DDR1 mutants that are not phosphorylated upon collagen incubation. (B–D) Wild-type or mutant DDR1b constructs were transiently expressed in HEK293 cells, either alone, or co-expressed as indicated. Cells were stimulated with collagen I or collagen IV for 90 min at 37°C. Aliquots of cell lysates were analysed by reducing SDS-PAGE and Western blotting. The blots were probed with phospho-specific Abs, as indicated, and re-probed with anti-DDR1. *, non-specific band. (C, D) Co-expression was performed with different amounts of DDR1b-Y513F expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa).