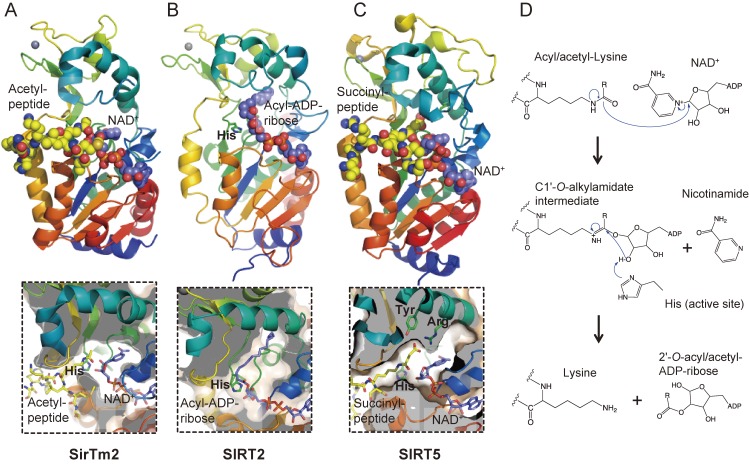
Figure 5.
Crystal structures and catalytic mechanism of sirtuin family proteins. (A) SirTm2 in complex with acetyl lysine peptide and NAD+ (PDB id: 2H4F), (B) SIRT2 in complex with acyl-ADP-ribose, an intermediate of the reaction between an acyl-peptide and NAD+ (4Y6Q), and (C) SIRT5 in complex with N-succinyl lysine peptide (3RIY). Active site histidine and substrates are represented as stick models, in which yellow, blue, red, and orange represent C, N, O, and P atoms, respectively. C atoms of NAD+ and acyl-ADP-ribose are in purple for clarity. Zn2+ ions are represented as space-filled spheres in grey. Close-up views of the active sites are shown in lower panels. (D) Catalytic mechanism of sirtuin family proteins. Acyl/acetyl-lysine residue is deacyl/deacetylated by nucleophilic addition of the acetamide oxygen to the C1′ position of the nicotinamide ribose. Nicotinamide and a deacylated/deacetylated peptide and a 2′-O-acyl/acetyl-ADP-ribose are final reaction products.