Figure 8.
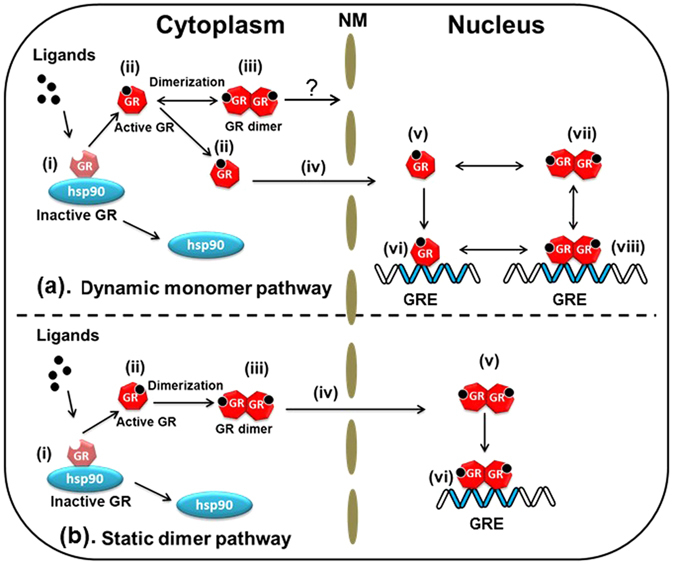
The proposed model for the pathways of glucocorticoid receptors. (a) The dynamic monomer pathway: (i) hGRα is localized to the cytoplasm as a complex or in free form in the uninduced state. (ii) hGRα is activated after ligand binding. Activated hGRα in the cytoplasm is in equilibrium between a monomer and dimer (iii) but transport of dimeric hGRα is unclear. (iv) Activated monomer hGRα relocates into the nucleus and is in both the free state (v) and monomer form, which can bind to a GRE as an unstable complex (vi). (vii) hGRα further dimerizes in the nucleus. (viii) The preformed dimer of hGRα associates with the GRE and other transcription factors. The dimer and monomer are not only distributed in the cytoplasm but also in the nucleus even after ligand binding; however, transport of hGRα is carried out in the monomeric form of hGRα. The concentration of hGRα in the nucleus can be controlled by changing the Kd of hGRα and GRE in the nucleus. (b) The static dimer pathway: (i) hGRα is localized to the cytoplasm as a complex or in free form in the uninduced state. (ii) hGRα is activated after ligand binding. Activated hGRα exists in the cytoplasm as a dimer (iii). (iv) In the dimer form, hGRα is translocated. The preformed dimer (v) of hGRα associates with a GRE and transcription factors (vi). The dimer of hGRα is distributed in both the cytoplasm and nucleus, but the monomer is found only in the cytoplasm. hGRα is transported in the dimer form. The concentration of hGRα in the nucleus can be controlled by the activity and functions of the NPC. hGRα: human glucocorticoid receptor α, hsp90: heat shock protein 90, GREs: glucocorticoid response elements, NM: nuclear membrane, NPC: nuclear pore complex
