Figure 6.
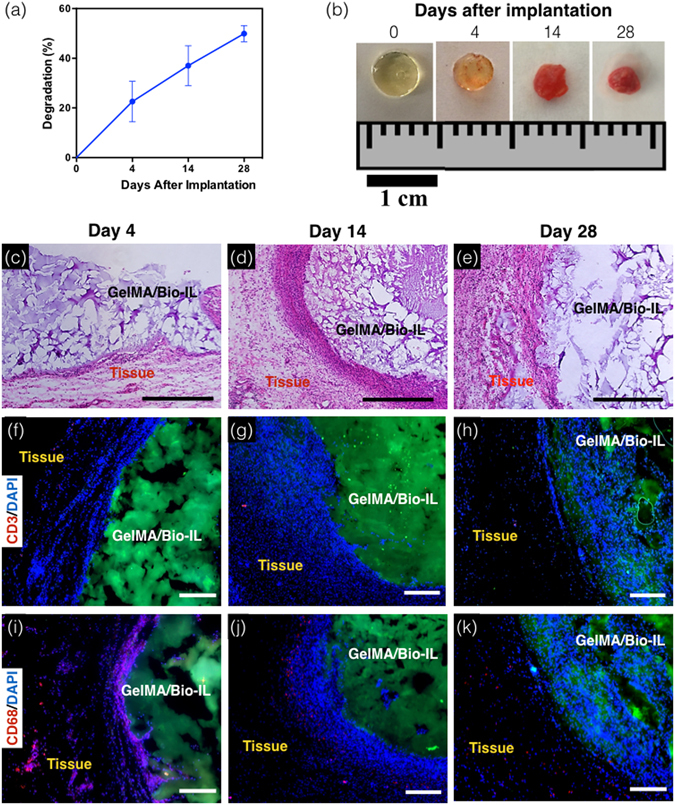
In vivo biodegradation and biocompatibility of GelMA/Bio-IL composite hydrogels using a rat subcutaneous model. (a,b) Evaluation of the in vivo degradation of GelMA/Bio-IL on days 0, 4, 14 and 28 post implantation (n = 4). (a) In vivo degradation of GelMA/Bio-IL hydrogels based on weight loss of the implants. The in vivo degradation profile of GelMA/Bio-IL hydrogels exhibited an approximately linear behavior throughout 28 days after implantation. (b) Images of the GelMA/Bio-IL composite hydrogels at 0, 4, 14, and 28 days after implantation. (c–e) Hematoxylin and eosin (H&E) staining of GelMA/Bio-IL sections after (c) 4, (d) 14, and (e) 28 days of implantation (scale bars = 500 µm). The H&E images shows a negligible amount of inflammatory cells. (f–h) Fluorescent immunohistochemical analysis of subcutaneously implanted GelMA/Bio-IL hydrogels showing no significant local lymphocyte infiltration (CD3) at days (f) 4, (g) 14 and (h) 28 (scale bars = 200 µm), and significant macrophage (CD68) presence at day (i) 4, which disappeared at days (j) 14 and (k) 28 (scale bars = 200 µm). Green, red and blue colors in (f–k) represent the GelMA/Bio-IL hydrogels, the immune cells, and the cell nuclei (DAPI). 1% VC, 1.5% TEOA, and 0.1 mM Eosin Y at 120 s light exposure were used to form the hydrogels. All hydrogels were synthesized using 15% final polymer concentration and 50/50 GelMA/Bio-IL ratio.
