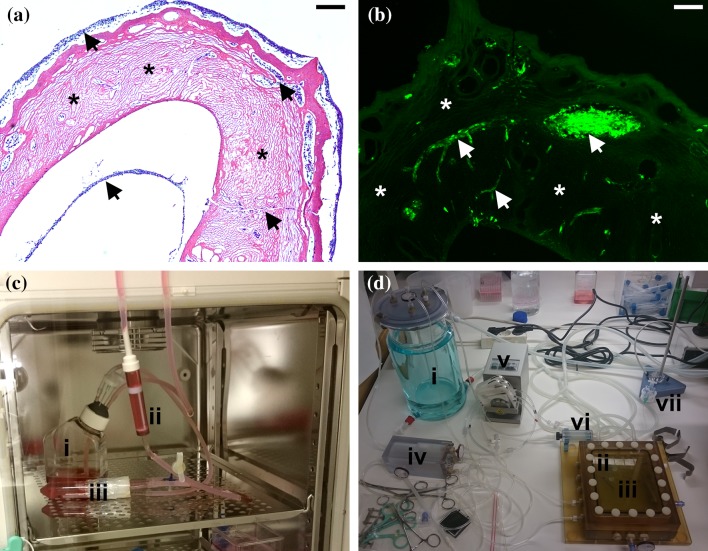
Figure 1.
Previously unpublished pictures of a Hematoxylin and Eosin stained section (a), and of a section with green fluorescent protein (GFP) labeled cells (b) from a recellularized whole rat uterus scaffold that was kept for 7 days in vitro after recellularization with about 300 million rat GFP-labeled bone marrow derived mesenchymal stem cells (MSCs). The scaffold was generated by a decellularization protocol based on perfusing the ionic detergent sodium deoxycholate and deionized H2O sequentially for 5 days. After recellularization, the engineered uterus construct was kept submerged in media that circulated in a closed, homemade, perfusion bioreactor which was maintained in a 37 °C humidified chamber supplemented with 5% CO2 (c). Note the vast cell-free scaffold areas in (a, b), and that cells mainly localized around the outside and on the luminal side of the scaffold (a), and in isolated pockets within the scaffold (b). (c) Picture of the homemade bioreactor used for the particular experiment shown in (a, b). Note that this particular system did not provide any extra oxygen supply to the media. We have now invested in a highly sophisticated perfusion bioreactor system from Hugo Sachs Electronic—Harvard Apparatus GmbH (jacketed psu moist chamber with tubing heat exchanger type 834/10) which gives us much better conditions for 3D-cell culturing, including temperature regulation, media oxygenation and pressure measurements (d). All animal experiments were approved by the Animal Ethics Committee in Gothenburg, Sweden. We are currently optimizing our recellularization techniques using this innovative system which hopefully also will extend our culturing times and reduce the contamination prevalence which has been a significant problem. Scale bar 200 µm. i, media cistern; ii, bubble trap; iii, organ perfusion site and reservoir; iv, oxygenator; v, peristaltic pump (not shown in c); vi, media heat exchanger; vii, pressure measure device.