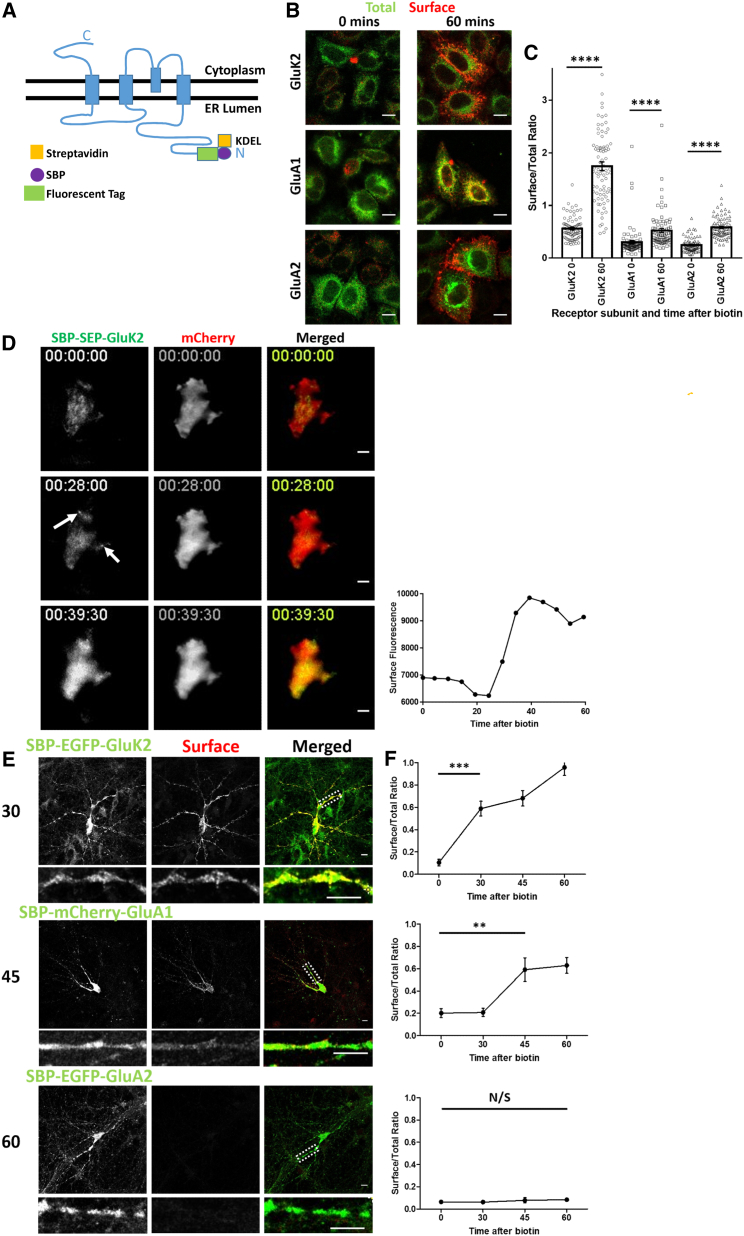
Figure 1.
Construction and Validation of RUSH Glutamate Receptor Subunits in HeLa and Primary Hippocampal Neuronal Cells
(A) Schematic of a RUSH ionotropic glutamate receptor subunit. SBP, streptavidin-binding peptide.
(B) Representative confocal images of the AMPAR subunits SBP-mCherry-GluA1 and SBP-EGFP-GluA2 and the KAR subunit SBP-EGFP-GluK2 in HeLa cells. Receptors are retained in the ER (0 min) and synchronously released by biotin addition, allowing trafficking to the cell surface (60 min after biotin addition). Total, green; surface anti-SBP, red.
(C) Quantification of the data represented in (B); three independent experiments, n = 80 cells/condition. ∗∗∗∗p < 0.0001, Welch’s t test.
(D) Representative still frames of the TIRF microscopy video (Figure S1B; Movie S1), showing the time course of trafficking and analysis of cell surface delivery of SBP-SEP-GluK2 after biotin addition. Arrows indicate sites of exocytosis. Quantification of surface delivery over time is also shown. See also Figure S1B.
(E) Representative confocal images of primary hippocampal neurons showing the differential secretory pathway trafficking rates of SBP-EGFP-GluK2, SBP-mCherry-GluA1, and SBP-EGFP-GluA2 containing KARs and AMPARs, respectively. Surface-expressed receptors were visualized using anti-SBP (red) at the indicated times (minutes) after biotin release. White boxes positioned on the merged panels indicate the region of the zoom panel.
(F) Quantification of the data shown in (E); three independent experiments, n = 20–24 for each receptor per time point. ∗∗∗p < 0.001, ∗∗p < 0.01, Welch’s t test.
Scale bars, 10 μm.