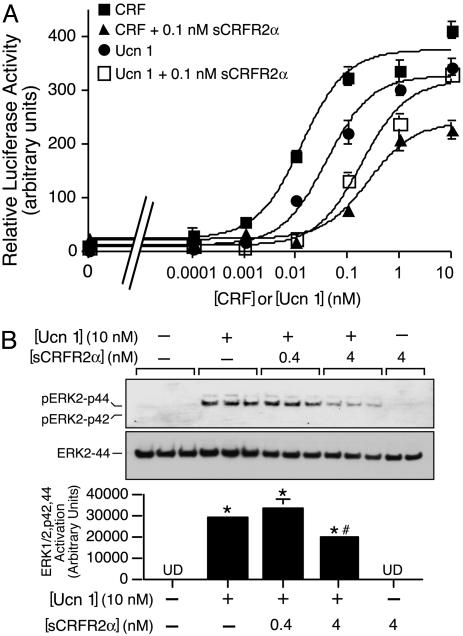
Fig. 5.
sCRFR2α protein interferes with the induction of cAMP and mitogen-activated protein kinase signaling mediated by Ucn1 or CRF. (A) Activation of cAMP response element-luciferase reporter by Ucn1 or CRF with or without sCRFR2α preincubation in 293T cells transiently transfected with mouse CRFR2α. Luciferase reporter containing a fragment of the cAMP response element promoter of the EVX1 gene was cotransfected into 293T cells with CRFR2α expression vectors. Luciferase activity was measured after 4-h treatment with 0.0001–100 nM Ucn1 or CRF, in the presence or absence of 0.1 nM sCRFR2α. Assays were normalized to cotransfected β-galactosidase activity. The mean of six replicates from one experiment are shown in the graph. (B) Equilibrated CATH.a cells were treated with 10 nM Ucn1 with or without 0.4 or 4 nM sCRFR2α. After 5 min of receptor stimulation, cell lysates were harvested and subjected to SDS/PAGE immunoblot analysis by using phosphorylated ERK1/2-p42,44 antibody and ERK2-p44 antibody. ERK activation was calculated by normalizing the levels of phosphorylated ERK1/2-p42,44 to total ERK2-p44. The means of triplicates from one experiment are shown in the graph. *, P < 0.05 vs. vehicle treatment; #, P < 0.05 vs. Ucn 1 treatment. UD, undetected.